2024 AIChE Annual Meeting
(100b) Nylon 6 Depolymerization: Reactor Design, Catalysts, Kinetics, Process Modeling, and Data Analytics
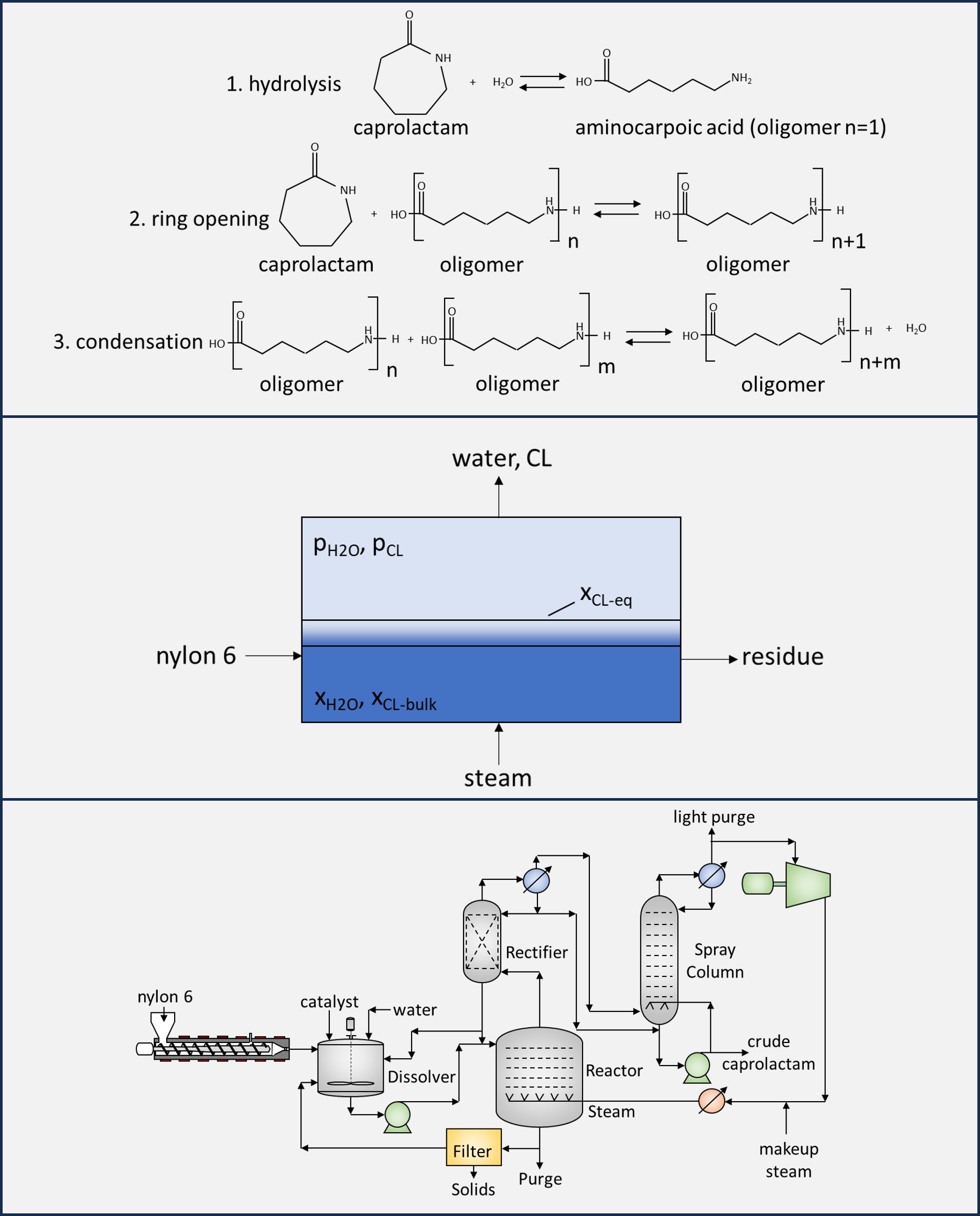
The depolymerization of nylon 6 to caprolactam can be divided into three different reaction types; (1) liquid-phase hydrolysis where nylon 6 melt is mixed with water at a high enough pressure that the reaction is completely liquid phase to produce a mixture of caprolactam and oligomers. (2) steam stripping where nylon 6 melt is contacted with superheated water vapor and formed caprolactam is stripped into the vapor phase. (3) solvent-free depolymerization where nylon 6 melt is held under vacuum and formed caprolactam is removed in the vapor phase. Each reaction type has fundamental differences and offers certain advantages and disadvantages.
Nylon 6 depolymerization processes can be uncatalyzed but can also use various catalysts, such as phosphoric acid, sodium hydroxide, or can use new catalysts such as early transition metal metallocenes. We discuss the advantages and limitations of each catalyst. We describe common depolymerization byproducts. The primary depolymerization byproduct is the cyclic dimer of caprolactam, which is undesirable in the caprolactam product, but can be recovered and converted to caprolactam. Several other minor degradation byproducts are also known to form and must be separated in downstream purification processes.
We present a fundamental analysis of the depolymerization kinetics. The reaction kinetics of nylon 6 are unique because of the parallel reactions that occur during depolymerization. Two primary reversible reactions are a condensation reaction, which impacts the chain length of the melt and the average molecular weight, and the ring-opening reaction, which impacts the rate of caprolactam evolved directly from the melt. The reverse-ring opening reaction rate which forms caprolactam is proportional to the concentration of amine end groups in the melt, which is a function of the average molecular weight or chain length of the melt. Thus, the condensation reaction and ring-opening reaction rates must be well defined to properly model nylon 6 depolymerization.
We use the kinetic models built for nylon 6 polymerization in Aspen Plus as the basis for our depolymerization models, because the primary kinetics responsible for polymerization are the same for depolymerization. We describe some of the challenges and modifications required for the depolymerization kinetic model and demonstrate the ability of the model to reflect experimental data for all three reaction types and for different catalysts. We also indicate where uncertainties exist in the modeling assumptions and how future studies can be developed to resolve these uncertainties.
We employed advanced data analytics and machine learning techniques to preprocess experimental data, effectively identifying and excluding process outliers. This enhanced the data's integrity, which is crucial for accurate kinetic estimation. Our approach incorporated hybrid modeling to produce pertinent experimental data, culminating in the development of a data-driven hybrid model for the reactor, tailored to the available limited experimental dataset.
Our review of nylon 6 depolymerization patents and development of a robust kinetic models for uncatalyzed and catalyzed reactions allows us to develop comprehensive process models for the nylon 6 depolymerization process with different reaction types and reactor configurations. We demonstrate different process intensifications and reactor configurations that can reduce overall reactor size and process energy demand. Furthermore, we leveraged data-based reduced order models to refine the energy optimization of the complex depolymerization process, achieving a more precise and computationally efficient optimization outcome.
Consequently, our research synergistically fused elements of reactor design, catalysis, kinetics, process modeling, and data analytics to finely tune the nylon depolymerization process. This holistic integration paves the way for a more sustainable and efficient recycling of nylon polymers for different reactor configurations.