2023 AIChE Annual Meeting
(87g) Insight into Electrochemical Batch Reactor for Phosphorus Recovery Using Mathematical Modeling
Introduction of mathematical continuum modeling into the study of electrochemical phosphorus recovery phenomena could play a crucial role in the design and optimization of reactors for this emerging recovery approach. It allows researchers to predict the behavior of the system under different conditions and to identify critical points in the reactor that can affect its performance. Mathematical models can also help reduce the cost and time required for experimentation and enable the analysis of phenomena that are difficult to observe experimentally.
In this study, a 2-D mathematical model of an electrochemical reactor was developed using COMSOL Multiphysics software. The model was used to investigate the hydrodynamics of the reactor, including impellers, electrochemical reactions, mass transfer of electroactive species, and phosphorus recovery. The model was solved numerically using the finite element method. The hydrodynamics of the reactor were investigated using the k-epsilon turbulence model, which is widely employed in literature to simulate the fluid flow and mixing behavior of the reactants. The electrochemical reactions that occur in the reactor were modeled using the Butler-Volmer equation at the cathode and the Tafel equation at the anode. The Butler-Volmer equation describes kinetics, while accounting for the concentration of electroactive species, the exchange current density, and the overpotential. On the other hand, the Tafel equation describes kinetics when the reaction rate is not controlled by the transport of reactants to the electrode surface. Boundary conditions for the potentials were a grounded counter electrode (anode) and applying -1.3 vs Ag/AgCl(sat.) to the working electrode (cathode). Mass transfer of electroactive species, such as phosphorus and oxygen, was modeled using Fick's second law of diffusion and the convective transport equation. The model also considered the migration of ions due to the electric field, which can affect the distribution of species inside the reactor.
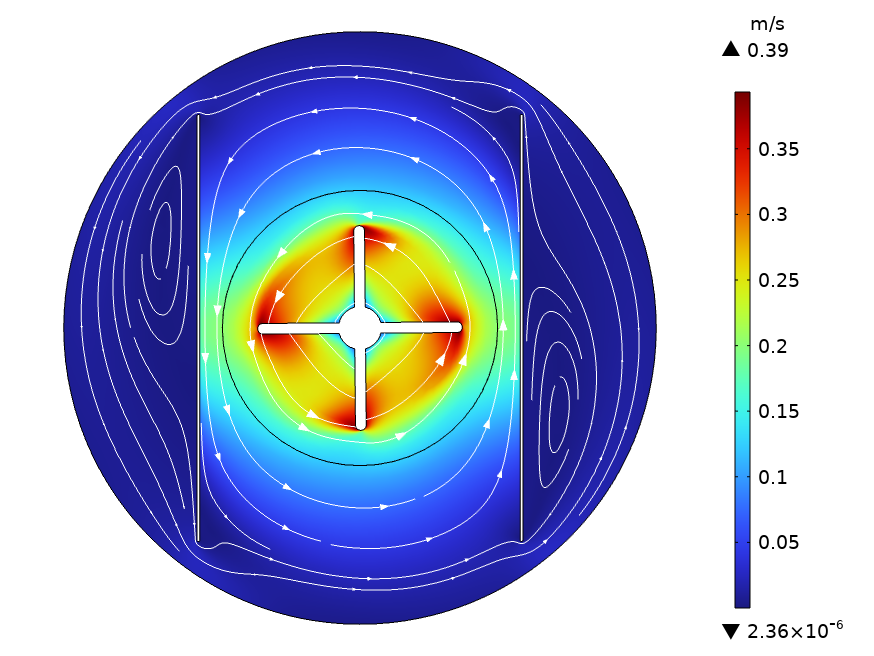
Preliminary results indicate that although the impellers can create turbulence and affect the local conditions around the electrodes, the backside of the electrodes is weakly affected by impeller rotation (Figure 1) which may adversely affect the mass transfer rate and electrochemical reactions. These results will be integrated with chemical and electrochemical reactions and then presented at the meeting, thus providing insight into the reactor behavior and options for optimization.
Figure 1: Flow field in a batch electrochemical reactor with impeller speed of 200 RPM, and at t = 21 s
Reference:
Bagastyo, A. Y., Anggrainy, A. D., Khoiruddin, K., Ursada, R., Warmadewanthi, I., & Wenten, I. G. (2022). Electrochemically-driven struvite recovery: Prospect and challenges for the application of magnesium sacrificial anode. Separation and Purification Technology, 288, 120653. https://doi.org/10.1016/j.seppur.2022.120653