2023 AIChE Annual Meeting
(689h) Base Enhanced Activation of Phosphorous-Containing Zeolites
Authors
Han Chen - Presenter, University of Massachusetts Amherst
Jason Gulbinski, University of Massachusetts Amherst
Choongsze Lee, University of Minnesota - Twin Cities
Tarnuma Tabassum, University of California, Santa Barbara
Michael Tsapatsis, Johns Hopkins University
Wei Fan, University of Massachusetts - Amherst
Susannah L. Scott, University of California, Santa Barbara
Dionisios Vlachos, University of Delaware - Catalysis Center For Ener
Stavros Caratzoulas, University of Delaware
Omar Abdelrahman, University of Massachusetts Amherst
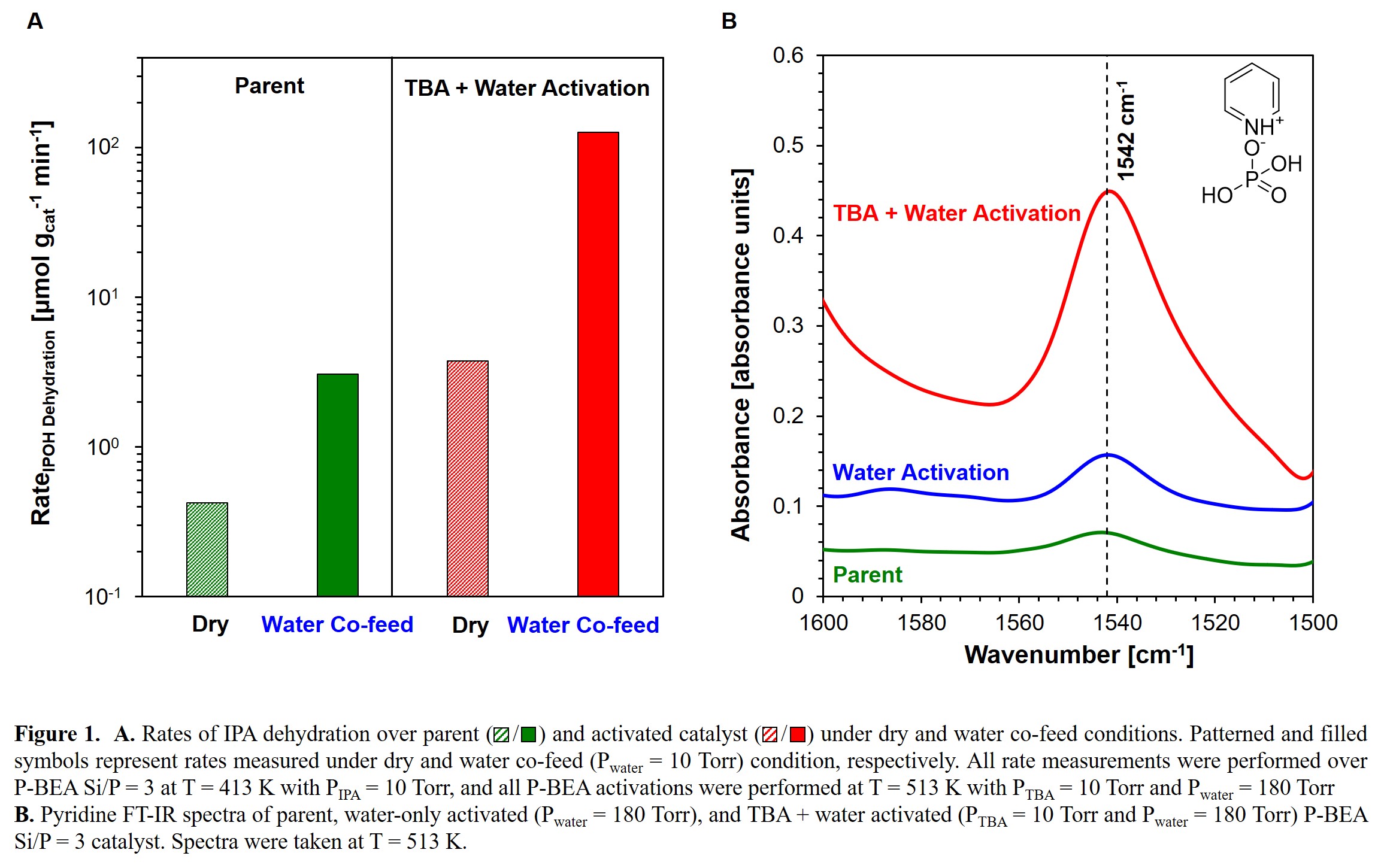
Phosphorus-containing catalysts have showed extraordinary selectivities to the desired products in several biomass upgrading conversions, e.g. dehydra-decyclization of cyclic ethers, due to their weaker acid strengths as compared with traditional solid acid catalysts.1 However, one limitation of these weakly acidic catalysts is their low catalytic activity. Therefore, catalyst that overcomes the activity limitation while maintaining a high product selectivity is expected. Herein, we demonstrate that the catalytic activity of phosphorus-impregnated zeolite (P-BEA) is enormously enhanced in the presence of water and basic molecules like alkylamine, as probed by alcohol dehydration and alkylamine Hofmann elimination. While treatment with water alone increased the rate of isopropanol dehydration (~7×), co-feeding a sufficiently basic molecule such as tert-butylamine (TBA) led to a ~ 300 factor rate increase (Figure 1A). A combination of in-situ kinetic titration and infrared characterization revealed that the Brønsted acid site density increased by two orders of magnitude when the catalyst was treated with TBA and a high partial pressure of water Pwater (Figure 1B). Such enhancement was found to be completely reversible by annealing the catalyst in helium flow, and the degree of rate enhancement was found to be positively correlated with the water partial pressure in the vapor phase feed after the initial activation. 31P solid state NMR and XANES revealed phosphorus species with various degrees of oligomerization on the activated P-BEA, demonstrating the activity enhancement was due to the hydrolysis of highly polymerized phosphorous species.
1. Abdelrahman, O. A.; Park, D. S.; Vinter, K. P.; Spanjers, C. S.; Ren, L.; Cho, H. J.; Vlachos, D. G.; Fan, W.; Tsapatsis, M.; Dauenhauer, P. J., ACS Sustainable Chem. Eng. 2017, 5, 3732-3736.