2023 AIChE Annual Meeting
(688e) A Microkinetic Framework to Understand the Promoting Role of Ba on Co-Ce Catalyst for Hydrogen Production Via Ammonia Decomposition
Authors
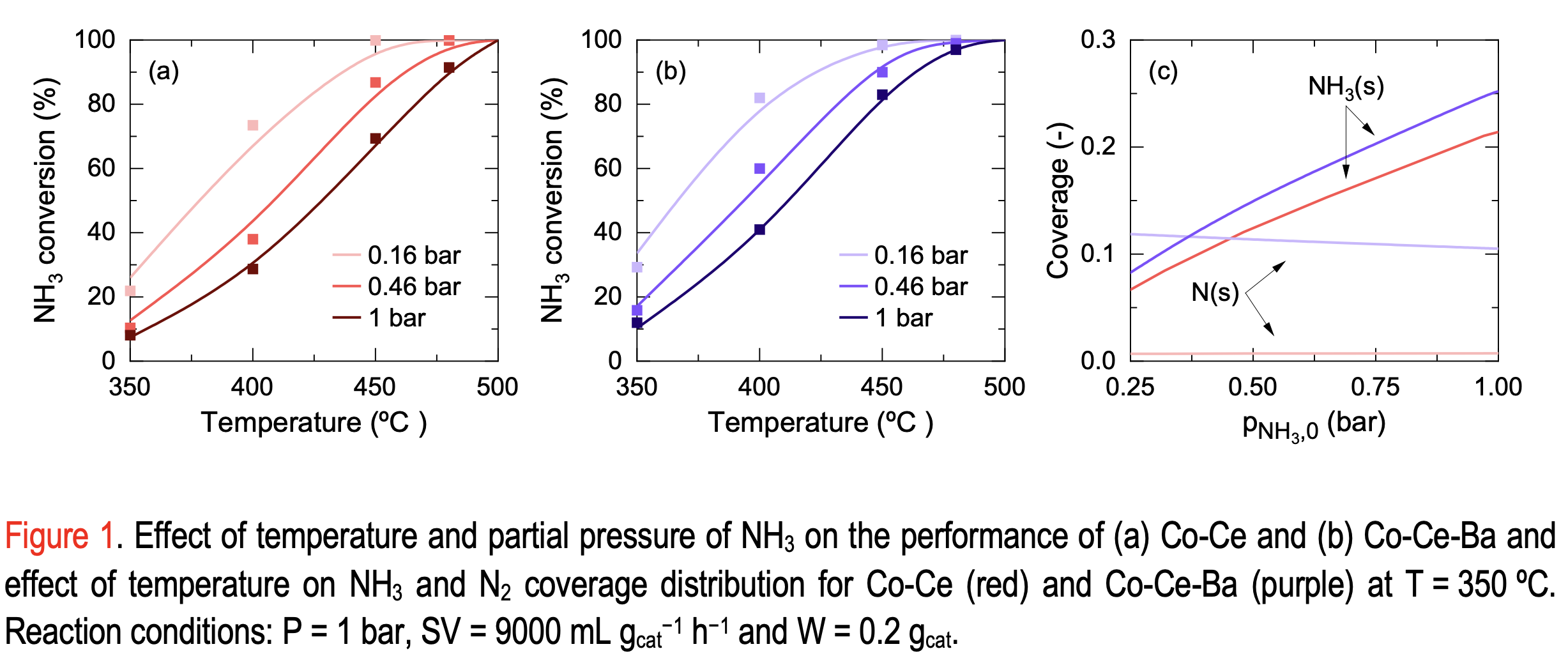
The present work explains the kinetic contribution of Ba using microkinetic rate expressions that offer additional information about the steps comprising the overall ammonia decomposition process. Combining experiments performed over a wide range of operating conditions for two Co-Ce catalysts, (with and without Ba) and the enthalpy-entropy constraints for the overall reaction, microkinetic rate parameters were optimized for both catalysts. By comparing the reaction path analyses in addition to the predicted catalytic species coverages for each catalyst, the kinetic role of Ba as a promoter is uncovered. Figure 1a-b shows the experimental and predicted NH3 conversions over various temperatures and NH3 partial pressures, exhibiting goodness of fit. In Figure 1 c, where the NH3 and N2 coverages are shown for the full range of NH3 partial pressures, it can be seen that the surface coverages intercept for the Co-Ce-Ba catalyst at low NH3 partial pressures while for Co-Ce they do not. Such a variation changes the elementary reaction rates, implying a change in the RDS or possibly the presence of two limiting steps for the given range. Thus, explaining the difference in activity between the promoted and non-promoted catalyst.