2023 AIChE Annual Meeting
(682g) Effects of TiO2 Structure on the Methane Partial Oxidation over IrOx/TiO2 Nanomaterials
Authors
Helena Hagelin Weaver - Presenter, University of Florida
Li-Yin Hsiao, University of Florida
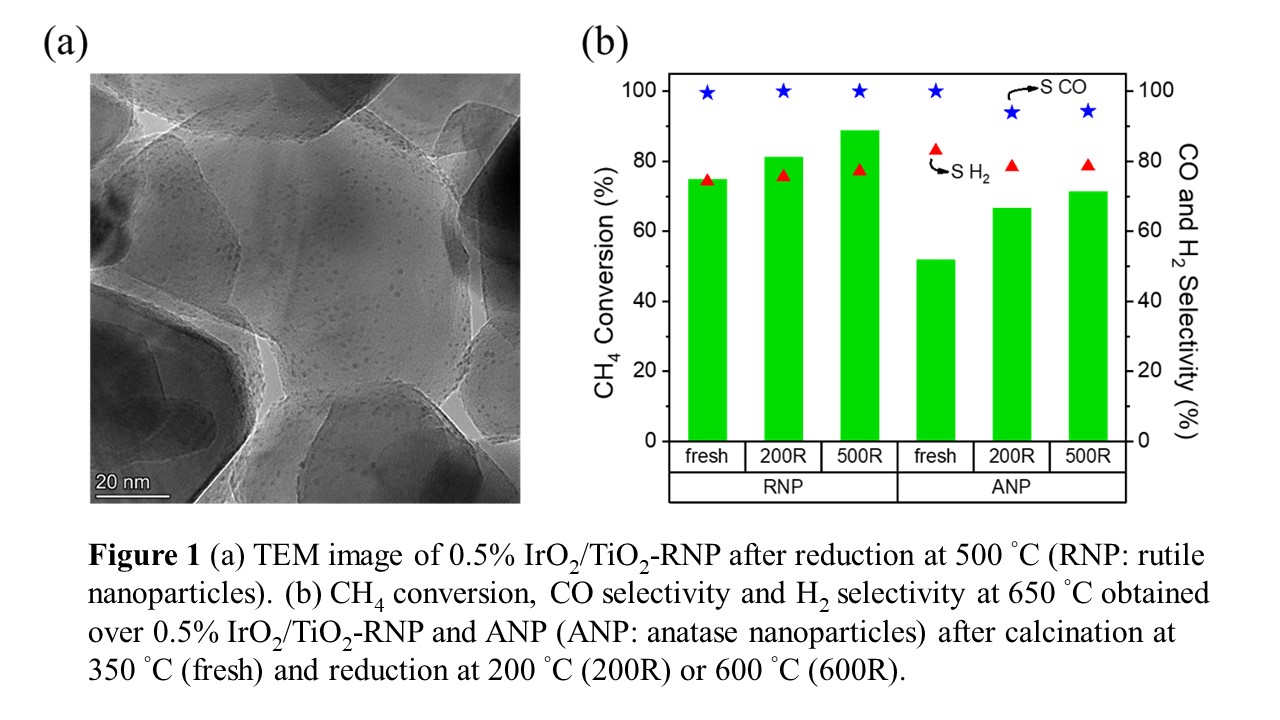
Partial oxidation of methane (POM) is an exothermic alternative pathway to produce synthesis gas (syngas), a mixture of CO and H2, compared to the endothermic steam reforming of methane. Noble metals supported on various metal oxides, such as TiO2, and Al2O3, are active and stable catalysts in the methane partial oxidation reaction, and IrO2/TiO2 is a particularly interesting combination with high selectivity to syngas. Despite the potential for IrO2/TiO2 in the POM reaction, few articles report on the differences in metal-support interactions between iridium and three main crystal phases of TiO2, namely rutile, anatase, and brookite phase. To determine how the crystal phase of TiO2 influences the activity and selectivity in the POM reaction, we synthesized IrO2/TiO2 catalysts with four different TiO2 supports. Iridium was deposited onto the TiO2 supports using the urea deposition-precipitation method, and calcination in air at 350 °C resulted in the IrO2/TiO2 catalyst. The catalytic performance of the IrO2/TiO2 in the methane partial oxidation reaction was then evaluated as a function of pretreatment conditions. Reduction at 200 °C is expected to reduce only the IrO2 to metallic Ir, while reduction at 500 °C can also reduce some of the TiO2, dependent on the crystal structure of the TiO2. Reducing condition can alter the Ir-TiO2 interactions, which in turn can influence the catalytic properties of IrO2 under the reaction conditions. The IrO2 on rutile TiO2 nanoparticles after 500 °C reduction (Figure 1(a)), exhibits the best CH4 conversion (88.8%) as well as 100% CO selectivity at 650 °C, while the worst performing catalyst is supported on anatase TiO2 (Figure 1 (b)).