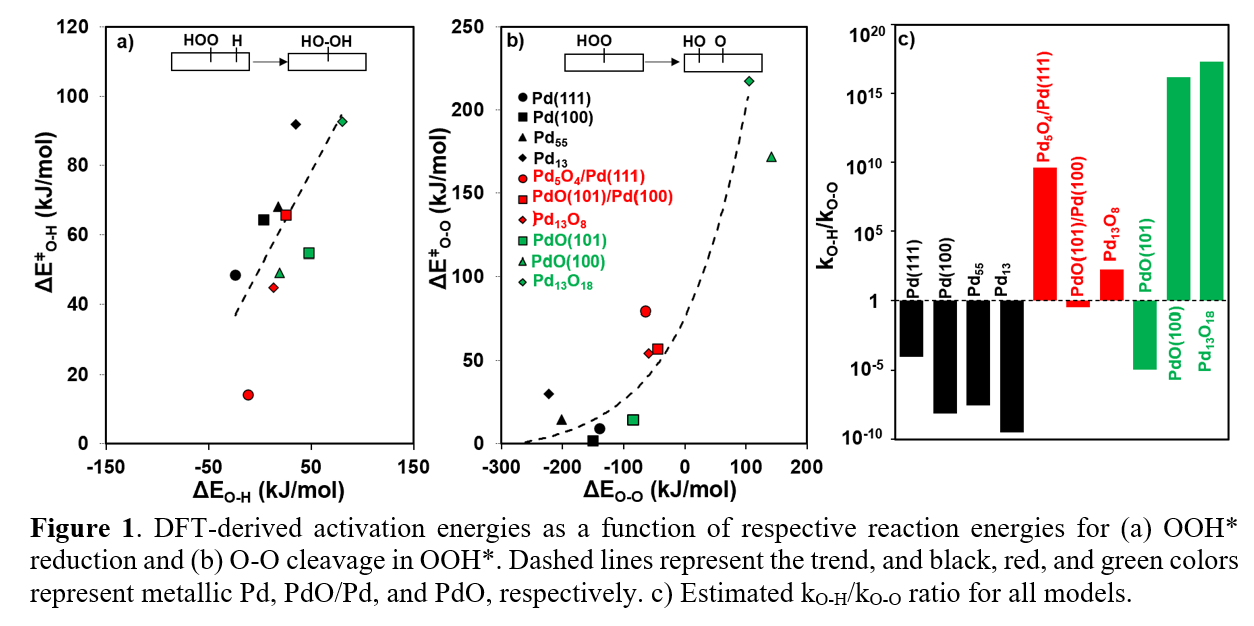
Direct H2O2 synthesis from H2 and O2 provides a green route to produce H2O2. Pd has been widely used for this process, although its commercialization is limited due to low H2O2 yields.1 This study aims to systemically assess the impacts of Pd-O coordination and particle sizes on primary H
2O
2 selectivities and H
2O
2 decomposition reactivities. Density functional theory (DFT) methods are implemented to calculate the rate constant ratios for
H2O2 formation (via OOH* reduction; k
O-H) and OOH* decomposition (via O-O cleavage; k
O-O) for metallic Pd, surface oxide, and bulk oxide models. The k
O-H/k
O-O ratio is much smaller for Pd
13 than for Pd(111) (10
-10 vs. 10
-4 at 300 K), indicating poorer primary
H2O2 selectivities for smaller particles. Yet, this ratio remained much smaller than unity for all Pd models. As the oxygen chemical potential increases, the Pd-Pd ensemble sites are perturbed by O atoms, which dramatically change their selectivities. The k
O-H/k
O-O ratio increased from 10
-4, 10
9 to 10
16 as Pd(111) oxidizes to Pd
5O
4/Pd(111) and PdO(100). This selectivity improvement, however, is very structure sensitive and the improvement remained minimal for surfaces that persistently contain Pd-Pd ensembles, such as PdO(101)/Pd(100) and PdO(101). Smaller PdO nanoparticles do not contain these facets and thus fully benefit from the selective enhancement. DFT-derived energy trends show that the catalysts with higher primary H
2O
2 selectivity are also less prone to H
2O
2 decomposition, leading to high H
2O
2 yields. Furthermore,
ab initio thermodynamics calculations are used to assess the relevant Pd phase in
O2 and H
2O
2/H
2O environments, revealing that smaller Pd nanoparticles are likely to present as PdO during H
2O
2 decomposition experiments. This study highlights how Pd transformation to PdO impacts H
2O
2 synthesis and decomposition rates and selectivities, which are sensitive to the Pd-O coordination environments and particle sizes.
References
- Flaherty, D.W. ACS Catal 2018, 8(2),1520â1527.