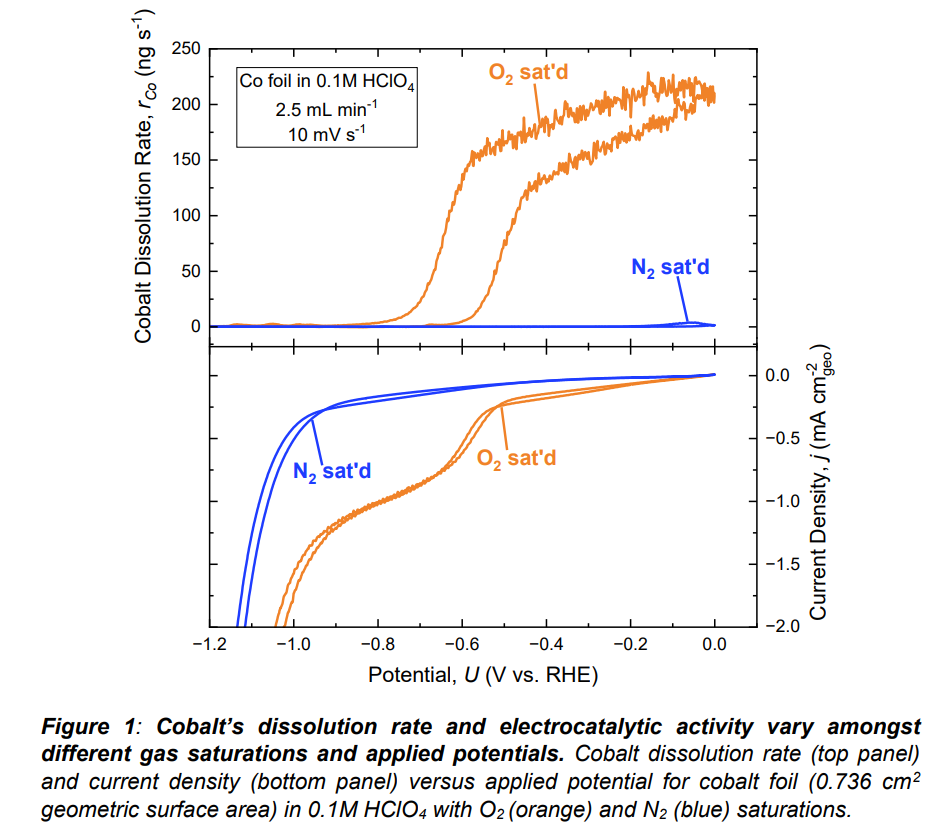
Cobalt (Co) thermodynamically has a large potential window of instability in acidic media that negatively impacts its usage in energy devices. In particular, leaching of Co from the PtCo/C catalyst cathode in commercial proton-exchange membrane fuel cells (PEMFCs)
limits the fuel cellâs overall performance and lifetime.
Minimizing electrode degradation while ensuring optimal performance of Co-based materials requires a more fundamental understanding of cobaltâs material stability in acidic media. In this work, we
simultaneous
ly monitor
the
electrocatalytic activity
and material stability of Co foil during the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) in 0.1M HClO
4 by coupling ICP-MS (inductively coupled plasma mass spectrometry) with an electrochemical flow cell (
known as on-line ICP-MS). To evaluate the role of catalysis on cobaltâs material stability, we compare Co dissolution in O
2-, N
2-, and H
2-saturated electrolyte in which we observe a ~140
-fold increase in dissolved Co under O
2 saturation relative to N
2/H
2 saturation at 0 V
RHE despite negligible faradaic current. These results indicate that the presence of O
2 destabilizes the Co surface in the absence of electrocatalysis. However, at potentials below cobaltâs ORR onset potential (-0.55 V
RHE @ -0.2 mA cm
-2), Co stabilizes and there is < 3.5 ng s
-1 dissolution at -0.8 V
RHE under all gas saturations despite the
magnitude of current density
differing by ~0.8 mA cm-2 between O
2 and N
2/H
2 saturation. These results
suggest that
electrocatalysis stabilizes the Co surface. To
further investigate the stabiliz
ation mechanisms of Co, we
employ electrochemical mass spectrometry (EC-MS) to measure the faradaic efficiency
towards HER. Altogether, this work provides important insights to better understand the degradation mechanism of Co in acidic media
under reaction conditions. Such understanding will motivate future studies to improve the stability of Co-based materials for a variety of applications, including fuel cells, electrolyzers, and lithium-ion batteries.