Catalytic combustion of CH
4 to CO
2 can reduce the environmental impact of CH
4Â emissions from point sources in the fossil energy sector by more than 96% while generating less NO
Âx compared to thermal combustion processes.
1 Pd/Al
2O
3 catalysts can oxidize CH
4 under stoichiometric (ð = 1) conditions, but under CH
4-lean (ð > 1), low temperature conditions, particularly in the presence of H
2O, it cannot achieve sufficient CH
4 conversion and remain stable. Pd-containing high-silica zeolites, such as Pd/SSZ-13 (CHA), outperform Pd/Al
2O
3 for complete CH
4 oxidation in the presence of H
2O and remain hydrothermally stable due to their hydrophobicity and superior durability.
2
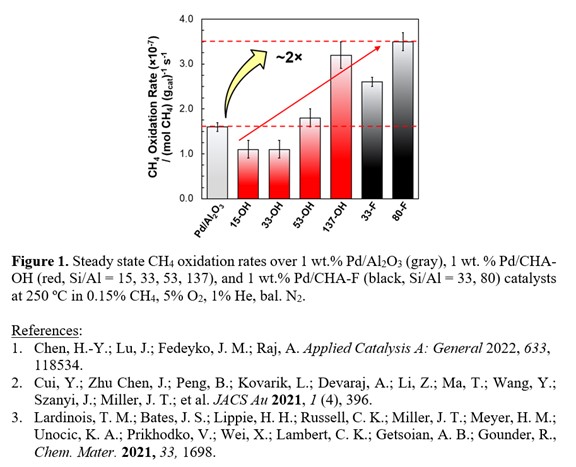
CHA zeolites (Si/Al = 15-137) were synthesized in both hydroxide (OH) and fluoride (F) media,3 ion-exchanged to 1 wt.% Pd, and then evaluated for CH4 oxidation activity before and after simulated aging for 1 h at 650 ºC under wet-lean conditions (0.15% CH4, 5% O2, 5% H2O, bal. Ar). A comparison of temperatures required to achieve 50% and 90% CH4 conversion (i.e., T50, T90) revealed that Pd/CHA (Si/Al > 33) demonstrates improved light-off activity and stability under dry and wet conditions compared to Pd/Al2O3. Decreasing T50 and T90 temperatures for Pd/CHA with increasing Si/Al molar ratios and results for Pd/CHAâOH (137) and Pd/CHA-F (80) of similar Si/Al ratio suggest that more hydrophobic zeolites improve low temperature performance and hydrothermal stability. Steady state CH4 oxidation rates measured on 1 wt.% Pd/CHA-F-80 at 250 ºC (0.15 % CH4, 5% O2, N2 bal.) were ~3.5 à 10-7 moles CH4 (gcat)-1 s-1, or ~2à greater than Pd/Al2O3 under similar conditions (Figure 1). Higher CH4 oxidation rates, higher apparent CH4 orders (~1), and lower activation barriers for Pd/CHA-F (Si/Al > 33) suggest that the nature of the active Pd is different on these hydrophobic zeolites with sites that can more easily activate CH4 compared to Pd/Al2O3.