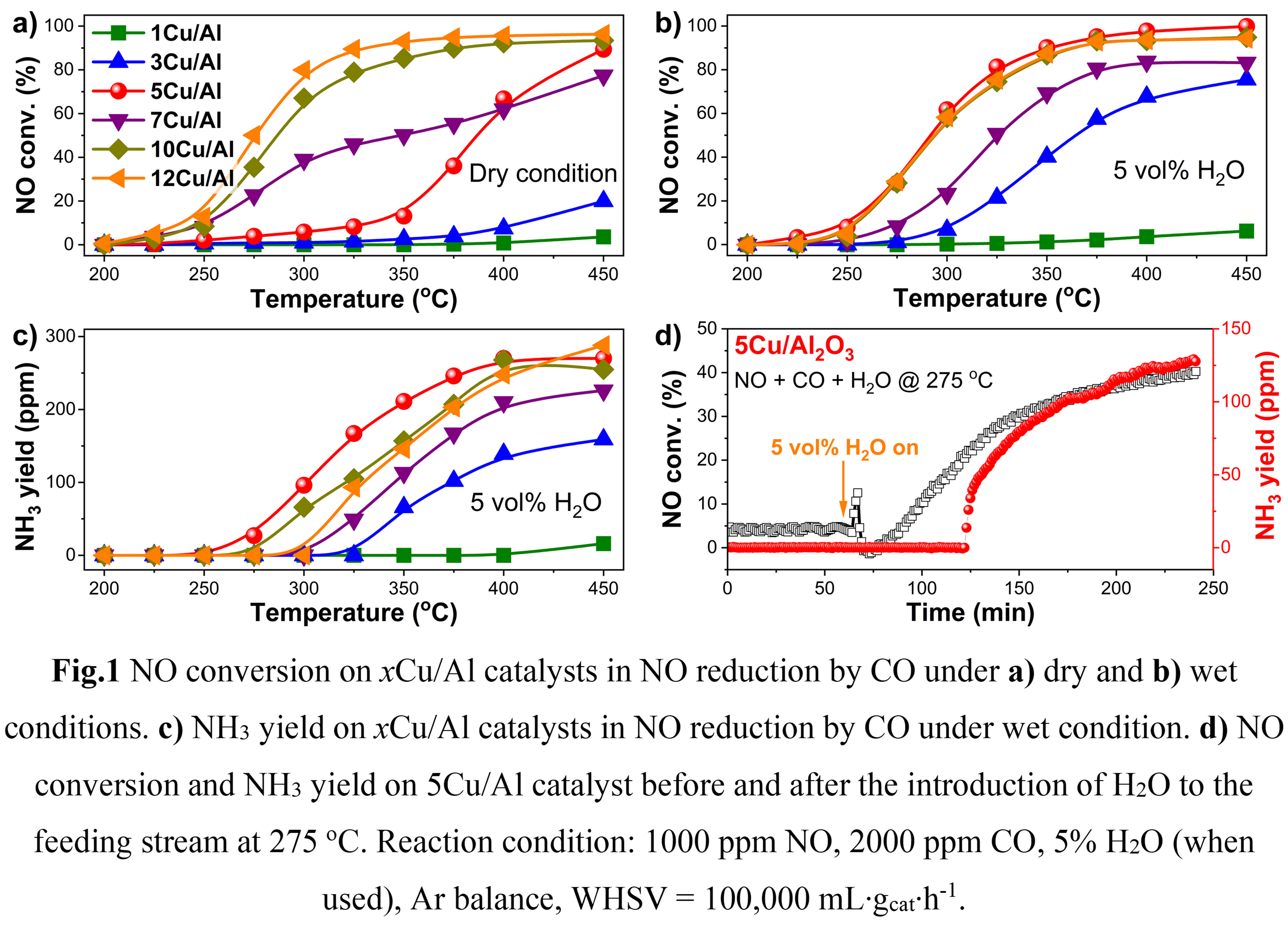
Based on our prior knowledge that CuO based catalysts exhibited superior catalytic performance in NO reduction by CO [1], CuO/Al
2O
3 serial catalysts with different CuO loadings (
x wt.% CuO/Al
2O
3, denoted as
xCu/Al) were synthesized using incipient wetness impregnation (IWI) method and evaluated for the possible NH
3 production from NO, CO and H
2O. With the increase of CuO loadings from 1 to 12 wt.%, monotonically increased NO removal efficiency was achieved on
xCu/Al catalysts under dry condition (
Fig. 1a). However, when 5 vol.% H
2O was added to the feeding stream, the NO conversion on 5Cu/Al increased significantly, achieving almost the same level as that on 12Cu/Al (
Fig. 1b). Abundant NH
3 was formed on
xCu/Al catalysts with the addition of H
2O to the feeding stream (
Fig. 1c and 1d). The NH
3 generation on 5Cu/Al was the most vigorous, which started at the lowest temperature (275
oC). The efficient conversion of NO to NH
3 and the possible reaction between NH
3 and NO should be the main reason for the noticeable enhancement of NO reduction efficiency on 5Cu/Al in the presence of H
2O. Unlike that isolated Cu ions or crystalline CuO particles were formed on CuO/Al
2O
3 catalysts with lower (⤠3 wt.%) or higher (⥠7 wt.%) CuO loadings, respectively, highly dispersed CuO
x clusters were formed on 5Cu/Al. The highly dispersed CuO
x clusters with superior redox performance could better facilitate the activation/dissociation of H
2O to react with NO and CO, forming NH
3 and CO
2. This work provides a new strategy for NH
3 production from two common air pollutants with the assistance of H
2O on economic CuO/Al
2O
3 catalysts under mild reaction conditions.
References:
[1] W. Tan, Y. Cai, S. Xie, J. Xu, K. Ma, K. Ye, L. Ma, S. N. Ehrlich, W. Zou, F. Gao, L. Dong, F. Liu. Chem. Eng. J. 456: 140807, 2023.