2023 AIChE Annual Meeting
(673b) Silver Single Atom Catalysts on Different Supports with Distinct Catalytic Performance for the Selective Oxidation of Ammonia
Authors
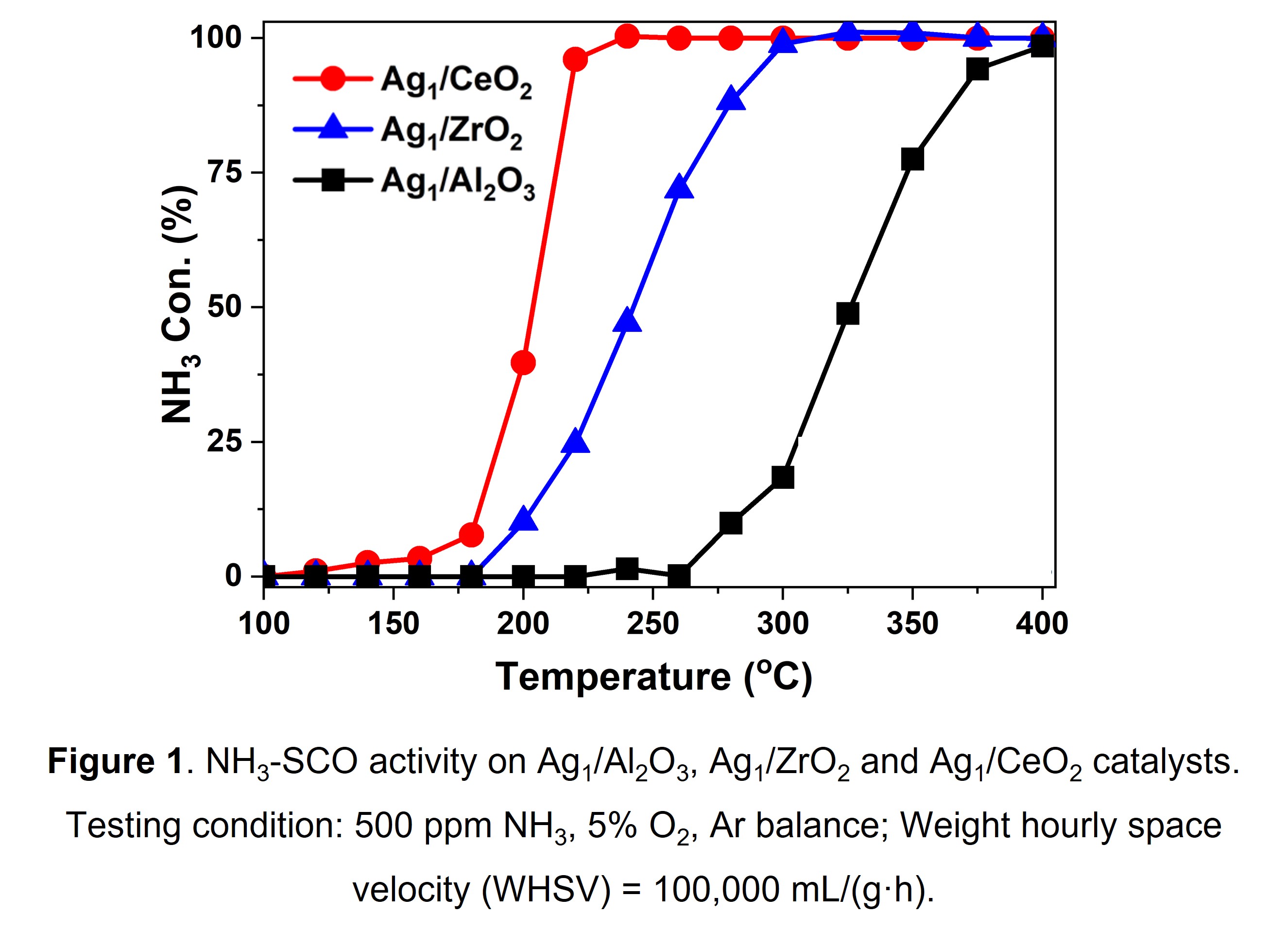
We herein report the preparation and application of Ag catalysts with 1wt.% Ag loading on three different supports including CeO2, Al2O3 and ZrO2 for NH3-SCO reaction. By means of TEM, UV-vis DRS and EXAFS, it was verified that Ag is present as single atom phase within all the three catalysts. Under different testing conditions with or without H2O, NH3-SCO activity on the catalysts decreased by the order of Ag1/CeO2 > Ag1/ZrO2 > Ag1/Al2O3 (Figure 1), with the Ag1/CeO2 catalyst performing the best. H2-temperature programed reduction (H2-TPR) results indicated that Ag1/CeO2 exhibited the best low-temperature reducibility. In accompanying density functional theory-based calculation, we believe that the greatest advantage offered by the Ag SACs on CeO2 is the formation of hydrogen bonds between NH3 and surface oxygen atoms, providing a drastically smaller activation energy barrier corresponding to shorter distances required for the hydrogen to dissociate.
References:
[1]. G.Y. Xu, Y. Zhang, J.G. Lin, Y.B. Wang, X.Y. Shi, Y.B. Yu, and H. He. ACS Catalysis 11(9): 5506-5516, 2021.