2023 AIChE Annual Meeting
(671a) Hydrogen Peroxide Electrosynthesis in a Strong Acidic Environment Using Cationic Surfactants
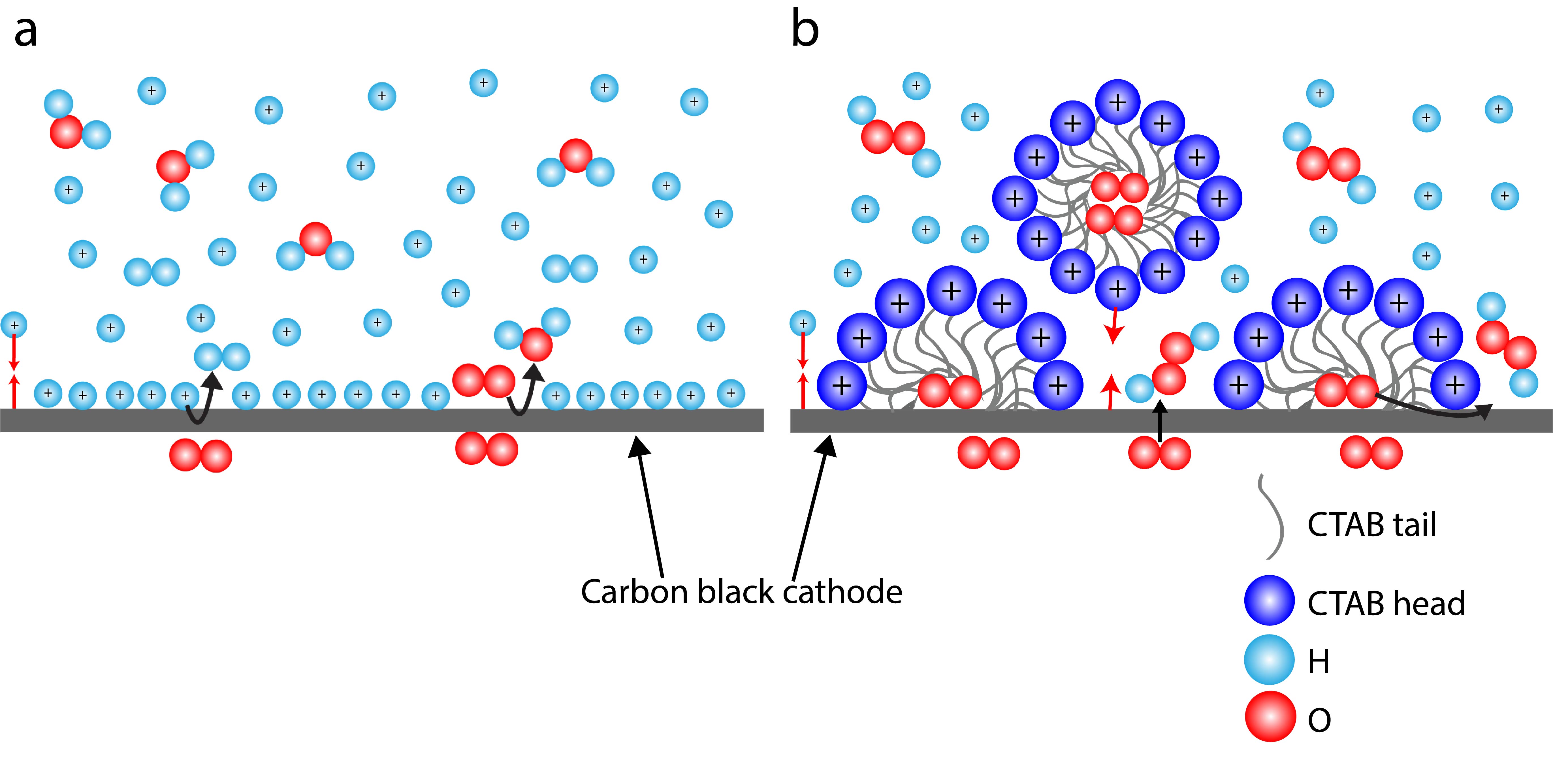
Hydrogen peroxide (H2O2) is an essential chemical utilized in disinfection, water treatment, chemical synthesis, and the paper and pulp manufacturing industry. Our group produces H2O2 via the two-electron oxygen reduction reaction (2e--ORR), which utilizes electricity from renewable resources. This technique has several advantages over the current industrial method for H2O2 synthesis, the anthraquinone process. No organic waste is produced; the infrastructure is not limited to a centralized location, reducing transportation costs; and the emissions are clean. Much of the H2O2 literature has focused on electrosynthesis in alkaline electrolytes as the higher pH environment favors the ORR over the hydrogen evolution reaction (HER). However, the implementation of H2O2 electrosynthesis in an acidic environment could help with the commercialization of practical H2O2 electrolyzers due to the superior stability of the in-situ generated H2O2 in low pH solutions and the compatibility with commercially-available proton-exchange membranes (PEMs). In order to exploit both high selectivity toward H2O2 product and high stability of the generated H2O2, we introduced cationic surfactants to a strongly acidic solution, which increased the H2O2 selectivity at low pH from 12% to 95% Faradaic efficiency (FE) under a current density of 200 mA cm-2. We employed in-situ surface enhanced Raman spectroscopy (SERS) and optical microscopy (OM) in order to understand the behavior of the electrode-electrolyte interface under electrolysis operating conditions. The results from our experiments demonstrate that the surfactant molecules become attracted to the electrode surface, likely displacing protons at the electrode-electrolyte interface, and thus promoting the ORR over HER. We also detected micelles in solution under OM imaging, which can facilitate O2 gas transport to the electrode surface, further favoring ORR conditions to produce H2O2.
Figure 1. Schematic portraying hypothetical electric double layer at cathode interface (a.) without CTAB and (b.) with CTAB.