2023 AIChE Annual Meeting
(669g) Re-Structuring of Interfacial Water into Strongly Hydrogen Bonded “Ice-like” Structures As the Unifying Descriptor for Improving Sluggish HOR/HER in Alkaline Electrolyte
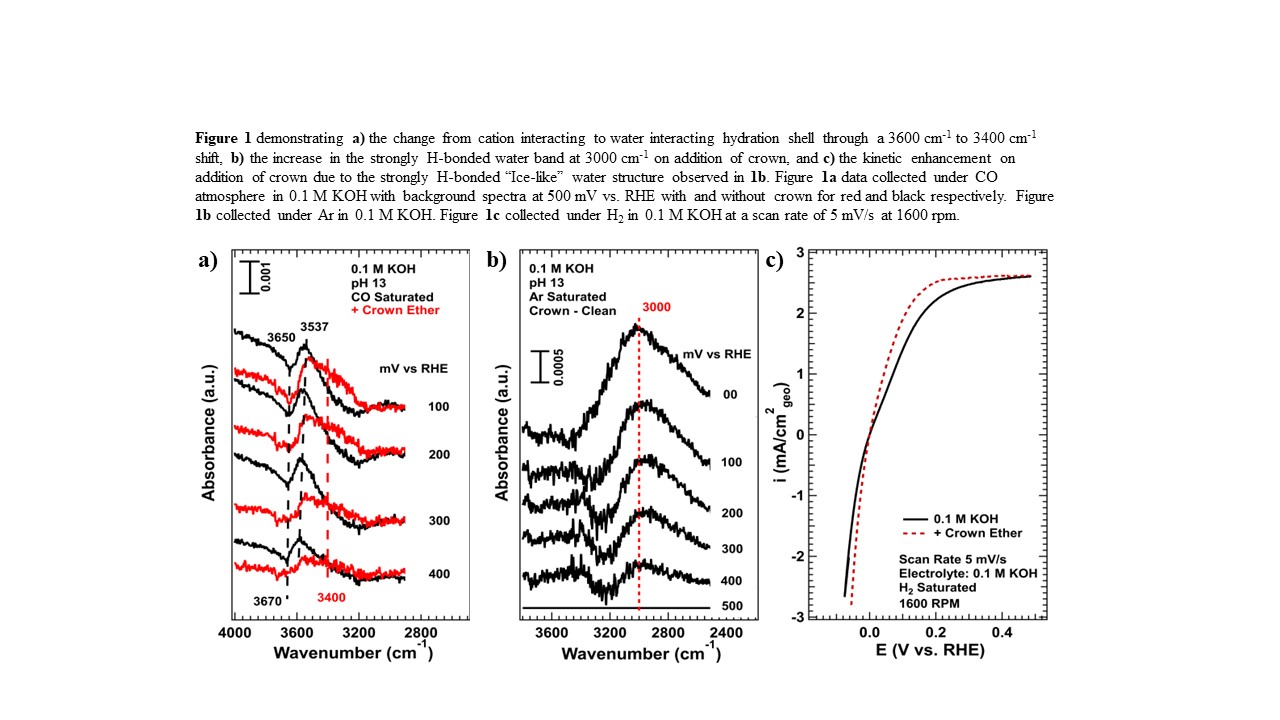
ATR-SEIRAS has been used to analyze the structure of cation hydration shells on Pt, where water interacts mainly with alkali metal cations through the increase of a 3600 cm-1 ν(O-H) stretch and weak interactions with surrounding interfacial water.3 Through chelation with crown ether, these hydration shells convert from strongly to weakly cation interacting, favoring instead hydrogen bonding with other water molecules, shown with increasing absorption bands at lower wavenumbers (3400 cm-1). This transition is accompanied by an increase in 3000 cm-1, assigned to strongly bonded âice-likeâ interfacial water. We show similar bands for other enhancing additives in the literature, showing that increasing âice-likeâ hydrogen bonded water is the unifying descriptor for improving PGM HOR/HER activity.
- Durst, J. et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7, 2255â2260 (2014).
- Monteiro, M. C. O., Goyal, A., Moerland, P. & Koper, M. T. M. Understanding Cation Trends for Hydrogen Evolution on Platinum and Gold Electrodes in Alkaline Media. ACS Catal. 11, 14328â14335 (2021).
- Yamakata, A. & Osawa, M. Cation-dependent restructure of the electric double layer on CO-covered Pt electrodes: Difference between hydrophilic and hydrophobic cations. J. Electroanal. Chem. 800, 19â24 (2017).