2023 AIChE Annual Meeting
(668a) Balancing Charge Carrier Kinetics and Photo-Absorption Capacity through Strain Engineering to Maximize Photocatalysis Under Visible-Light.
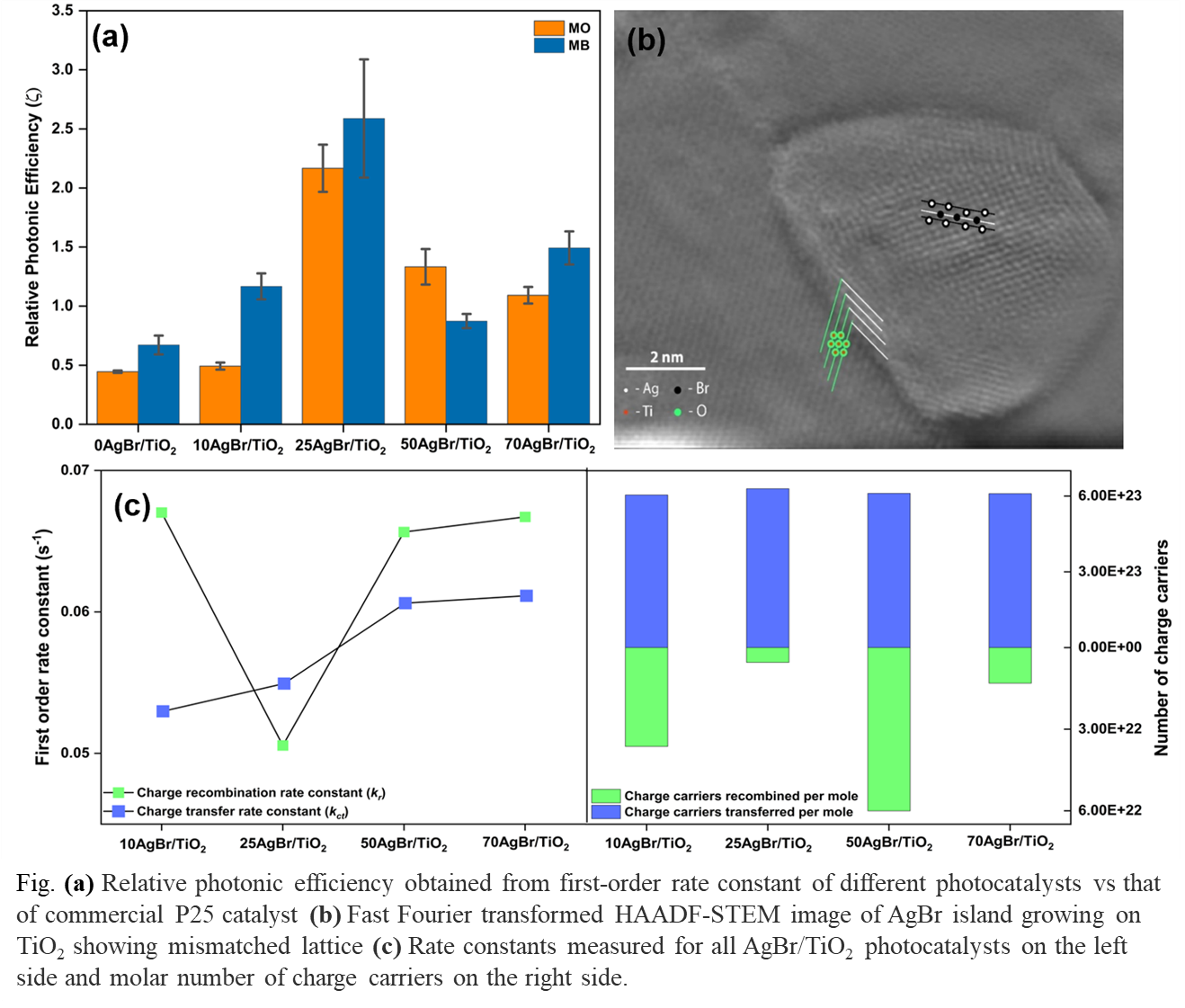
Addition of noble metal halides certainly transform TiO2 into a visible-light photocatalyst, but whether this transformation is due to a reduction in it's bandgap or a synergy of enhanced photoelectrical properties of TiO2 and noble metal halides, is a subject of debate. Furthermore, the optimized loading of noble metal halides at which best photocatalytic activity occurs has neither been correlated with a single material property in the literature consistently nor linked to the photogenerated charge carrier dynamics. In this study, we investigate the change in intrinsic lattice strain of TiO2 by loading different concentrations of AgBr; a popular photoactive plasmonic semiconductor which narrows the bandgap of TiO2 and makes the resulting nanocomposite, a visible-light active photocatalyst. XRD analysis revealed that beyond a maximum strain developed at an optimum AgBr loading level of 25wt.%, the grain boundary interface of TiO2 and AgBr nanoislands could no longer withstand the increased strain in their TiâTi, and TiâO bonds. A chain of thermodynamically governed crystalline phase transformation then begins within TiO2 which creates defect states in the band tails of anatase TiO2 thus lowering it's bandgap. Faster dye degradation kinetics corroborate the hypothesis that catalyst possessing maximum lattice strain show superior photocatalytic properties. Photoelectrochemical studies was used to quantify the rate of charge transfer (kct) and rate of recombination (kr) of photogenerated electrons and holes. These studies revealed that high lattice strain reduced the recombination of charge carriers by trapping e- within the transient interband tails of TiO2. At AgBr concentrations higher than 25wt.%, the kct was increased due to plasmonic resonance but ultimately kr overtook the net charge carrier flux decreasing the photocatalytic efficiency of TiO2. This work establishes the role of lattice strain in constructing high-performance plasmonic photocatalysts suitable for visible light application by balancing their optical and electrical properties.