2023 AIChE Annual Meeting
(667b) Tuning the Bulk Composition of Pt-Based High-Entropy Alloys for Improved Oxygen Reduction Activity
Authors
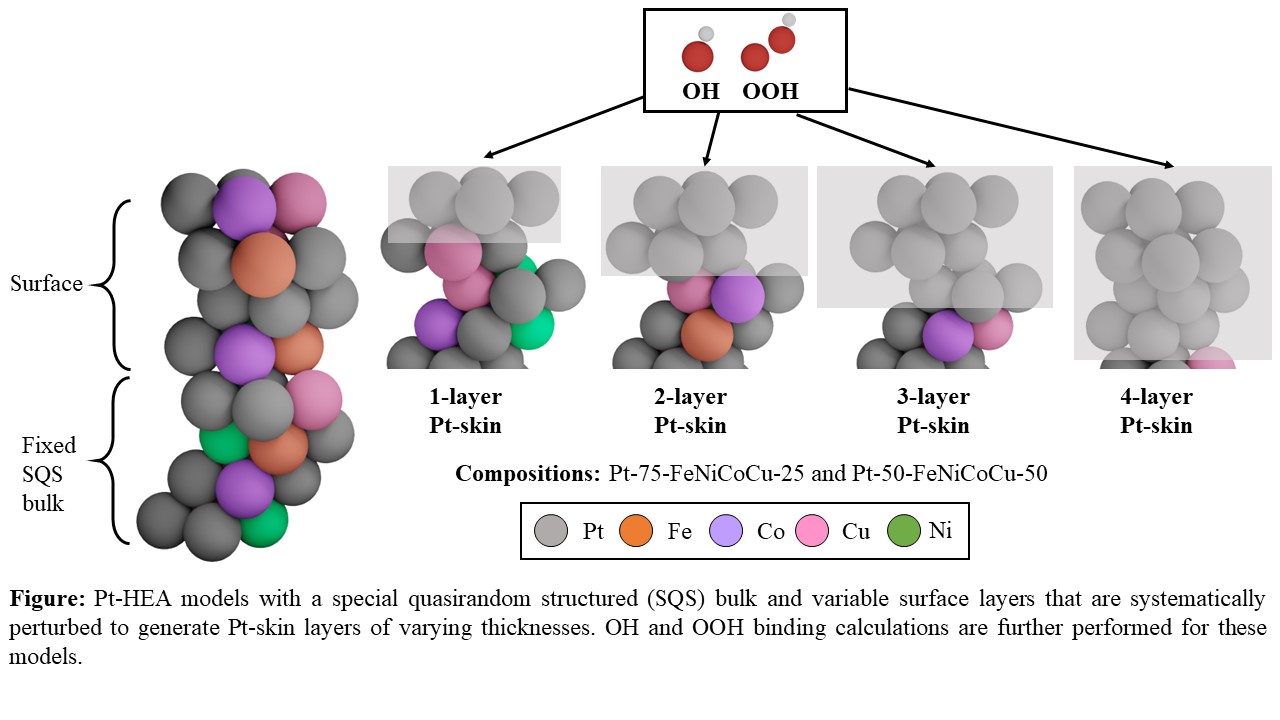
In this work, we present a first-principles analysis of Pt-based HEA catalysts with non-precious elements (Fe, Ni, Co, and Cu), which we have determined to be experimentally active for the ORR. Based on our experimental STEM-EDX resultsâwhich show that multilayer Pt-skins form in the near-surface region due to leaching of non-precious metalsâwe first probe the influence of ligand and ensemble effects on the activity by calculating binding energies of ORR intermediates like OH and OOH (using DFT) as a function of the number of Pt-skin layers on thousands of HEA active sites. Second, we examine the effect of near-surface strain relaxation on the ORR activity, and we find that the two effects compete with each other. Finally, we incorporate these effects into a theoretical activity calculation and find that the ORR activities of almost all the active sites follow the traditional ORR volcano relationship.
Based on these rigorous analyses, we rationalize bulk strain as a simple descriptor for the ORR activity and present a simplified strain-based volcano analysis to optimize catalytic activity. By mapping composition to strain using a Vegardâs law-type relation, we identify an HEA composition with optimal activity, synthesize it, and find that it shows a current density (per area) about 10 times that of Pt, thus validating our theoretical prediction. We close by discussing potential machine learning-based strategies to accelerate the design of HEAs.