2023 AIChE Annual Meeting
(664e) Asymmetrical C–C Coupling for Electroreduction of CO on Bimetallic Cu–Pd Catalysts
Authors
Hao Shen - Presenter, University of Illinois at Chicago
Chao Wang, Johns Hopkins University
Tim Mueller, Johns Hopkins University
Yunzhe Wang, Johns Hopkins University
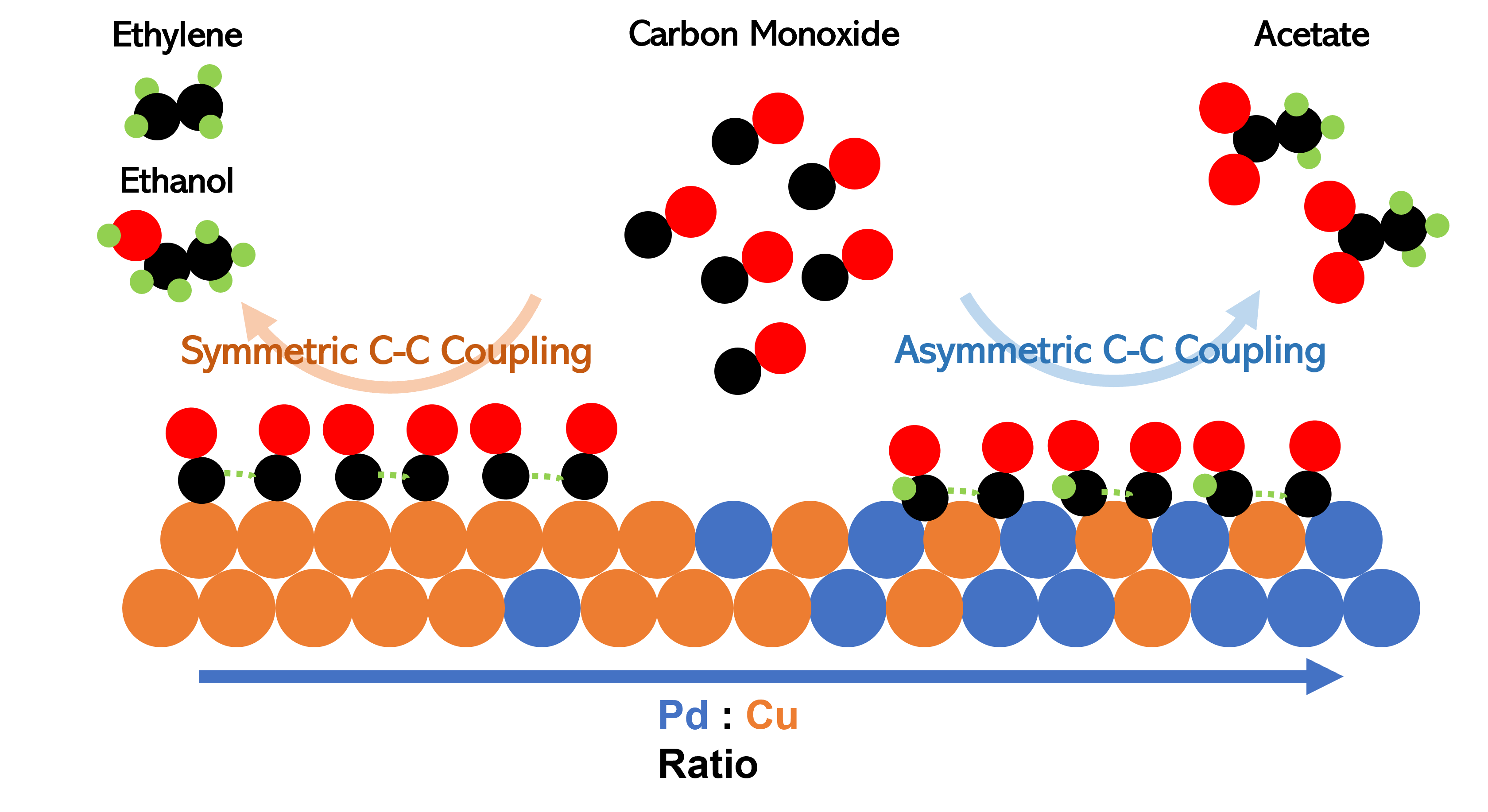
Electrochemical reduction of carbon dioxide (CO2) is promising for the mitigation of carbon emissions and building up carbon-neutral energy infrastructures. However, the undesired side reaction with CO2 has limited the practical implementation of alkaline electrolytes in CO2 electrolyzers. This challenge can be circumvented by the sequential electroreduction of CO2 to CO and then CO to C2+. Thus, electroreduction of carbon monoxide (CO) possesses great potential for achieving the renewable synthesis of hydrocarbon chemicals from CO2. We report here selective reduction of CO to acetate using CuâPd bimetallic electrocatalysts. Surfactant-free CuâPd nanocrystals were synthesized with control over the composition (Cu70Pd30, Cu49Pd51, Cu23Pd77) and applied for electrocatalytic studies by using gas-diffusion electrodes and flowing alkaline catholytes. High activity and selectivity are demonstrated for CO-to-acetate conversion with >200 mA/cm2 in geometric current density and >65% in Faradaic efficiency (FE). An asymmetrical CâC coupling mechanism is proposed to explain the composition-dependent catalytic performance and high selectivity toward acetate. This mechanism is supported by the computationally predicted shift of the *CO adsorption from the top-site configuration on Cu (or Cu-rich) surfaces to the bridge sites of CuâPd bimetallic surfaces, which is also associated with the reduction of the CO hydrogenation barrier. Further kinetic analysis of the reaction order with respect to CO and Tafel slope supports a reaction pathway with *COâ*CHO recombination following a CO hydrogenation step, which could account for the electroreduction of CO to acetate on the CuâPd bimetallic catalysts. Our work highlights how heteroatomic alloy surfaces can be tailored to enable distinct reaction pathways and achieve advanced catalytic performance beyond monometallic catalysts.