2023 AIChE Annual Meeting
(658f) Boosting Ethanol Selectivity in Electrocatalytic CO2 Reduction Reaction through Confinement of Intermediates in Au/Cu Tandem Catalyst
Authors
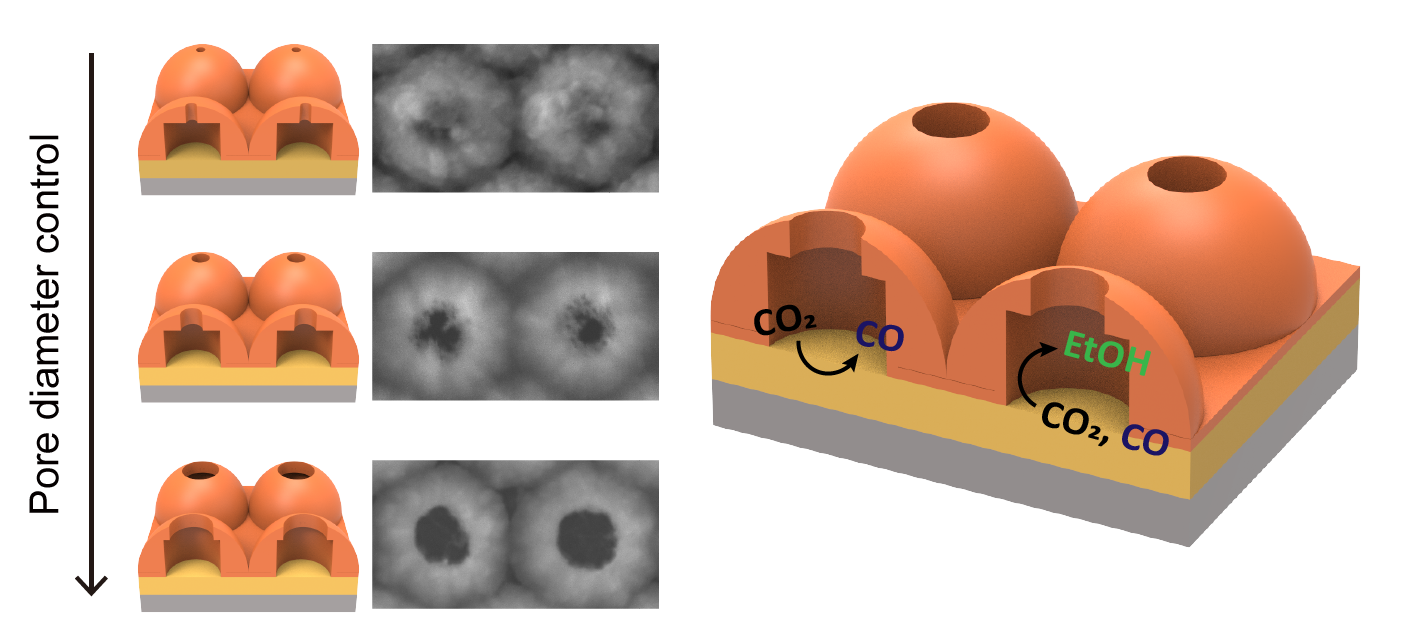
In this study, we aimed to investigate effect of each confined structure and tandem, and to maximize the local confinement effect by combining both confined and tandem structures. We designed a nanostructure consisting of two parts: a metal film layer on the bottom and a porous Cu layer on top. To investigate confined structure effect, Cu was used for metal film layer (Cubottom/Cupore) with different pore size. On Cubottom/Cupore catalyst, selectivity of EtOH was highly influenced by pore size, and highest on 50 nm of pore diameter. To investigate tandem effect, Au was used for metal film layer (Aubottom/Cupore), and combination of confined and tandem effects resulted in increased selectivity of C2+ products compare to Cubottom/Cupore, regardless of pore size. Especially, the selectivity of EtOH reached 50.8 % at -0.9 V vs RHE. The locally confined intermediates and reaction pathway in tandem porous catalyst was inferred through in-situ ATR-SEIRAS.
In summary, the novel nanostructure allowed us to identify the optimal confined structure dimensions for EtOH selectivity, resulting in significant improvement in EtOH selectivity on the tandem confined structure.