2023 AIChE Annual Meeting
(650d) Interfacial Engineering of Metal/Support Heterostructures for CO2 Electro-Reduction to C1 Products and Beyond
Authors
Lavie Rekhi - Presenter, NTU Singapore
Luan Q. Le, Nanyang Technological University
Asmee Prabhu, Nanyang Technological University
Kah Meng Yam, Nanyang Technological University
Tej Choksi, Nanyang Technological University
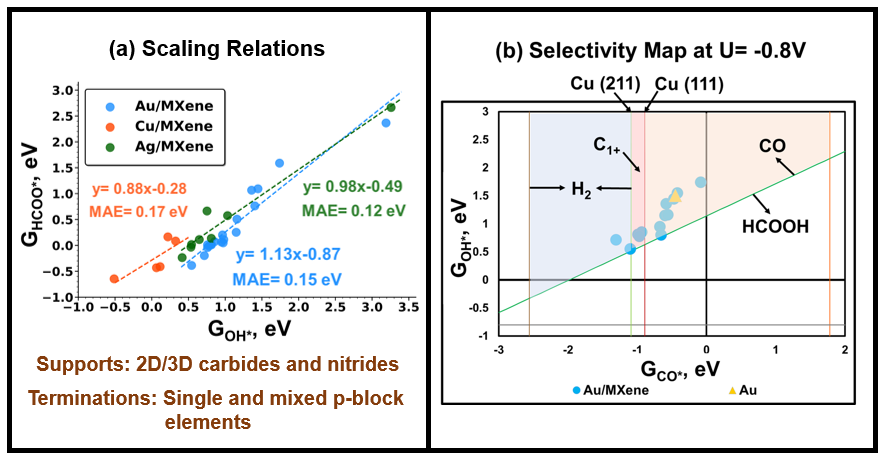
Controlling the selectivity of CO2 electro-reduction to one specific product has emerged as a long-standing challenge in catalysis. While strain, alloying, and nano structuring have been used to create active sites, tuning selectivity by manipulating the charged state of active sites is less studied. In our recent study, gold nanoparticles supported on InP and TiO2 were employed to showcase the impact of charged states of Au sites on the composition of syngas during electro-reduction of CO2 (Liu G. et al., ACS Catal. (2021)). Building upon this observation, we demonstrate how the selectivity of CO2 reduction to various products (e.g., syngas and C1+) can be influenced by manipulating the charged state of supported metal sites. Our set of materials comprises of low-strain (< 5%) epitaxial films and moiré patterns of Au, Ag, and Cu monolayers supported on 2D/3D transition metal carbides and nitrides. The surface terminations of supports include single and mixed p-block elements which induce distinct charge states on the metal sites. Based on a paradigm introduced by Tang et al. (Appl. Catal. B Environ. (2020)), we use adsorption energies of CO* and OH* as reactivity descriptors to classify the trends in selectivity. We construct metal-specific linear scaling relations between adsorption energies of reaction intermediates in CO2 electro-reduction and the reactivity descriptors. The scaling relations exhibit slopes that deviate from the bond order conservation principles observed in unsupported metallic systems. These scaling relations, when combined with free energy equilibrium conditions, are utilized to establish selectivity boundaries for different competing pathways, such as HCOO*/COOH* pathways and CH4/C2/H2 product formation. We present a novel approach for tailoring the selectivity of electrocatalysts for CO2 reduction by controlling interfacial charge transfer. Our model allows for quick evaluation of the selectivity of metal/support heterostructures, broadening the range of potential catalysts beyond Cu and its alloys.