2023 AIChE Annual Meeting
(646f) Understanding Hydrogenation of CO/CO2 Mixtures to Light Hydrocarbons over Ru-Co Single Atom Alloys Via Low-Temperature Fischer Synthesis.
Authors
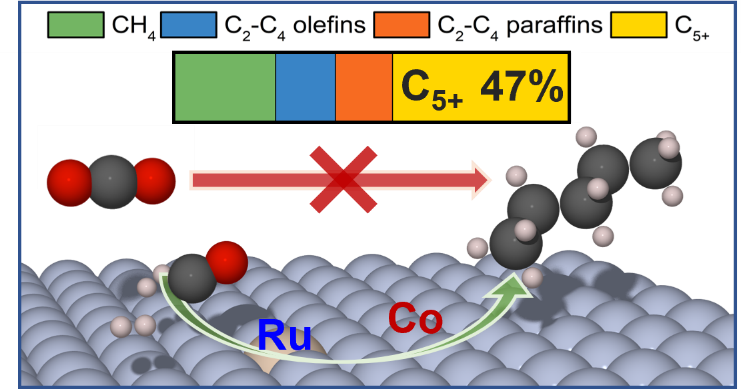
In this work, we employed Density Functional Theory (DFT) to understand the nature Ru-Co Single-Atom Alloy (SAA) catalysts, experimentally found by our collaborators to be active for low-temperature FTS. DFT-calculated segregation trends reveal that Ru single atoms are likely located on the surface, in accordance with experimental characterization. Furthermore, DFT-computed CO and CO2 adsorption energies confirm that CO2 does not participate in the hydrogenation chemistry, as it does not chemisorb on any of the catalytically relevant systems. Remarkably, Ru isolated atoms were not found to improve activity and selectivity towards the desired C5+ products, as observed in elementary reaction pathways of carbon coupling and C-H dissociation reactions. Instead, computed oxygen-vacancy formation energies show that the role of Ru is to promote the reduction of Co species, enabling catalytic activity for CO hydrogenation without pre-reduction. This work not only demonstrates the effective use of Ru promoters on Ru-Co SAA for low-temperature FTS, but also opens a new avenue for the exploration of other Co-based SAA catalysts for hydrogenation reactions.