2023 AIChE Annual Meeting
(625e) Efficient Cu-Based Catalysts for CO2 Hydrogenation to Methanol: An Exsolution Strategy with CuAl2O4 As Precursor
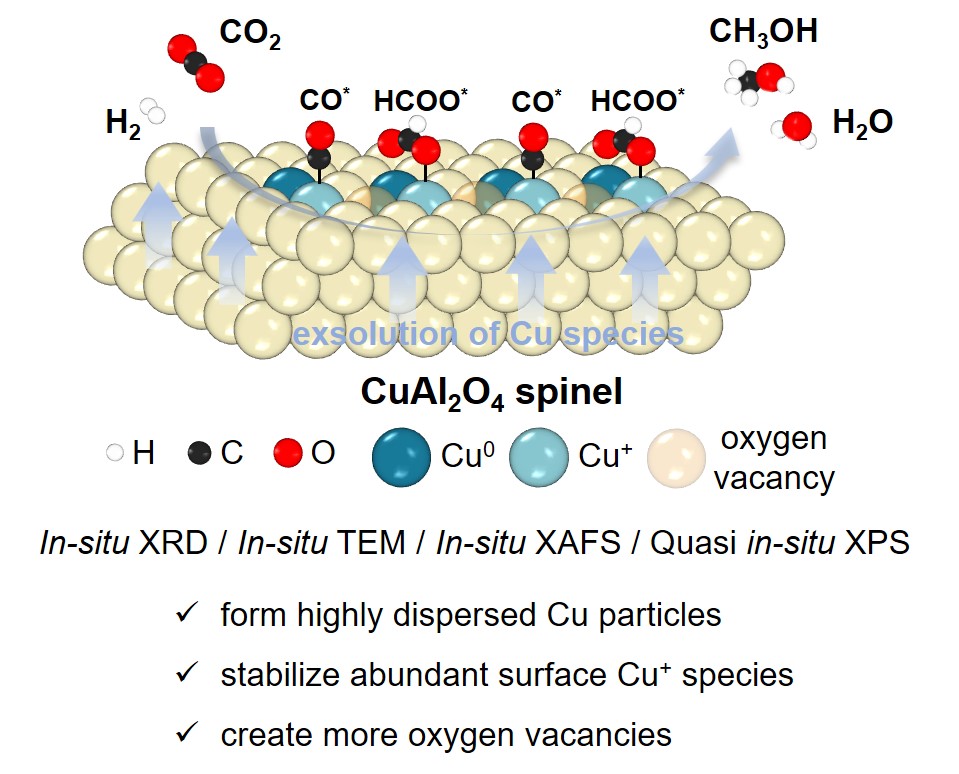
Catalytic conversion of carbon dioxide to methanol using green hydrogen produced from renewable energy is a potential process to achieve carbon reduction. Cu-based catalysts are of great interest due to their availability and high efficiency in hydrogenation reactions. For the rational design of Cu-based catalysts to further improve the reaction activity and space-time yield of methanol, it has been revealed that highly dispersed Cu particles, abundant oxygen vacancies, and reasonable surface Cu+ content are crucial for activating reactants and stabilizing reaction intermediates. This study focuses on the development of a selective exsolution method using CuAl2O4 spinel as precursor to regulate the dispersion and electronic structure of Cu species to obtain efficient Cu-based catalysts for CO2 hydrogenation to methanol. After a suitable reduction treatment, the CuAl2O4 catalyst shows enhanced CO2 conversion, better methanol selectivity, and 5.7 times higher methanol yield compared to the supported Cu/Al2O3 catalyst under the conditions of 240 °C and 3 MPa. The in-situ characterization of the exsolution process of CuAl2O4 demonstrates that highly dispersed Cu particles with an average particle size of 10 nm are formed. In contrast to the Cu/Al2O3 catalysts, abundant Cu+ species and oxygen vacancies are formed on the surface of CuAl2O4 due to the stronger metal-support interaction. It is expected to further improve the catalytic performance of Cu-based catalysts for CO2 hydrogenation to methanol by adding various metal oxides (i.e., ZnO or ZrO2) on the CuAl2O4 precursor and applying this exsolution strategy to construct copper-oxide interfacial sites while increasing the content of Cu+ species on the catalyst surface.