2023 AIChE Annual Meeting
(625a) Promoting Pdo Formation Via Pre-Exposure to Ambient Moisture
Authors
Tala Mon - Presenter, University at Buffalo
Junjie Chen, University At Buffalo
Eleni Kyriakidou, SUNY at Buffalo
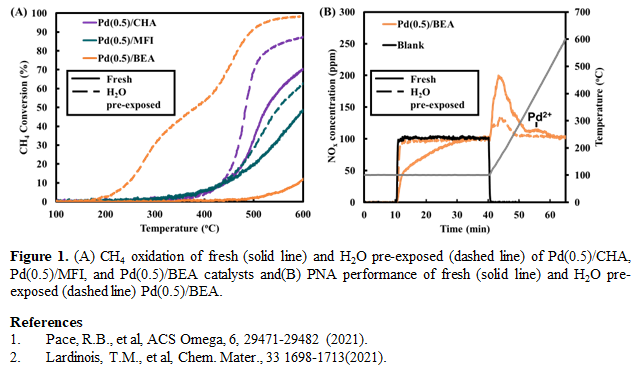
Pd speciation is essential to the investigation of Pd-based zeolite catalysts for both passive NOx adsorption (PNA) and methane (CH4) oxidation applications with ionic Pd and PdO as the active sites, respectively [1]. Herein, the effect of H2O exposure on Pd speciation is investigated at ambient temperature (25oC) and moisture (3.1% H2O) over Pd (0.5 wt.%) supported on small (CHA, 3.8Å), medium (MFI, 5-6Å), and large (BEA, 6-9Å) pore zeolites. 100 mg (250-500 µm) of catalyst was loaded in a packed bed micro-reactor (200 Lg-1h-1) and ionic Pd formation was promoted through a pretreatment at 750°C/1 h (20% O2/Ar) [2]. The catalysts were then treated with 3.1% H2O and 20% O2/Ar at 25oC/4 h to investigate the speciation of Pd after H2O exposure. CH4 oxidation (1500 ppm CH4, 12% O2/Ar, 100 - 600oC (5oC/min)) and PNA (100 ppm NO, 12% O2/Ar, 30 min adsorption at 100oC and desorption from 100-600oC (20 oC/min)) were used as probe reactions for titrating PdO and ionic Pd, respectively, before and after exposure to H2O. The CH4 oxidation performance of all studied Pd/zeolite catalysts improved after H2O exposure compared to the fresh catalyst performance. Specifically, the CH4 oxidation performance at 600oC improved by 85, 15, and 18% over Pd(0.5)/BEA, Pd(0.5)/MFI, and Pd/(0.5)/CHA, respectively, after H2O exposure (Fig. 1A). Moreover, the NOx:Pd ratio and thus, the NOx adsorption capacity, decreased by 94% and 32% after H2O exposure over Pd(0.5)/BEA (Fig. 1B) and Pd/(0.5)/CHA, respectively, indicating a decrease in ionic Pd. The decrease in ionic Pd species resulted in PdO formation (CO-DRIFTS and H2-TPR) through solvation of ionic Pd and aggregation to form PdO after oxidative dehydration. This work illustrates that Pd speciation should be taken into consideration when Pd/zeolite catalysts are pre-exposure to H2O under ambient conditions.