2023 AIChE Annual Meeting
(610d) Cancer Cell Migration in Physiologically Relevant Confining Microenvironments
Authors
Brent Ifemembi, Johns Hopkins University
Konstantinos Konstantopoulos, Johns Hopkins University
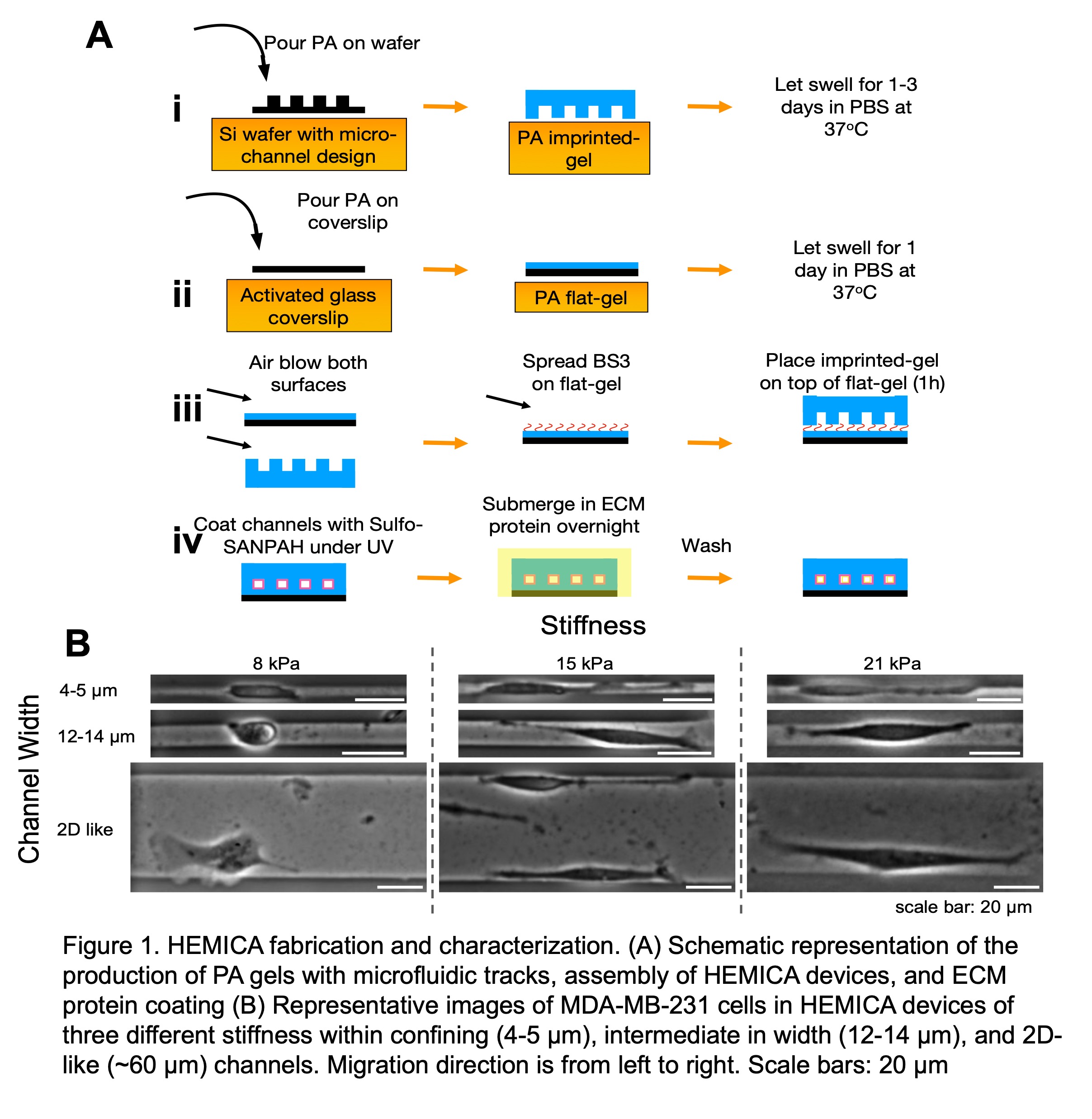
Cancer metastasis is responsible for over 90% of cancer-associated deaths. In the metastatic cascade, cells migrate from the primary tumor through confining microenvironments to form secondary metastases. To improve our tools for defeating cancer, we must advance our understanding of confined cancer cell migration in physiologically relevant microenvironments. Traditional in vitro devices to investigate confined cell migration are composed of Polydimethylsiloxane (PDMS), but this material does not fully reflect the physiological conditions that cancer cells experience in vivo. PDMS is not permeable to water and is an elastomer with a stiffness range of 1.3 and 3 MPa, much higher than the physiological tissue stiffness of 8-86kPa. In this study, we developed polyacrylamide (PA)-based microfluidic devices with tunable physiological stiffness (8 kPa to 21 kPa) and channel-like migration tracks ranging from 4 to 60 µm in width and 200 µm in length. With our novel hydrogel-encapsulated microchannel array (HEMICA) we can examine the importance of substrate stiffness in cancer cell migration. First, by performing innovative three-dimensional traction force measurements of spatially confined cells we discovered that increased substrate stiffness leads to increased traction forces in confinement. Secondly, we identified the key role that myosin-IIA (MIIA) contractility and integrin-beta-1 have on confined breast cancer cells in HEMICA, opposed to their marginal contributions in PDMS devices. Such differences are due to a reduction in RhoA activity and a smaller number of focal adhesions in compliant substrates. Lastly, we observed the role that ion transporters, such as the sodium hydrogen exchanger 1 (NHE1) have on confined migration in soft vs stiff substrates. We discovered that the previously proven front-rear polarization of NHE1 is abrogated as cells navigate softer substrates, but restored when cells move back to stiffer environments. By utilizing our innovative HEMICA device we have uncovered stiffness-dependent differences in confined cell migration, polarization, and gene expression that have not been observed in conventional in vitro settings and have demonstrated the importance of physiologically relevant microenvironments in regulating cell motility.