2023 AIChE Annual Meeting
(574g) Entropy Creation, Waste Work and Thermodynamic Efficiency of Galvanic Cells: Effects of Discharge Current and Environment Temperature
Author
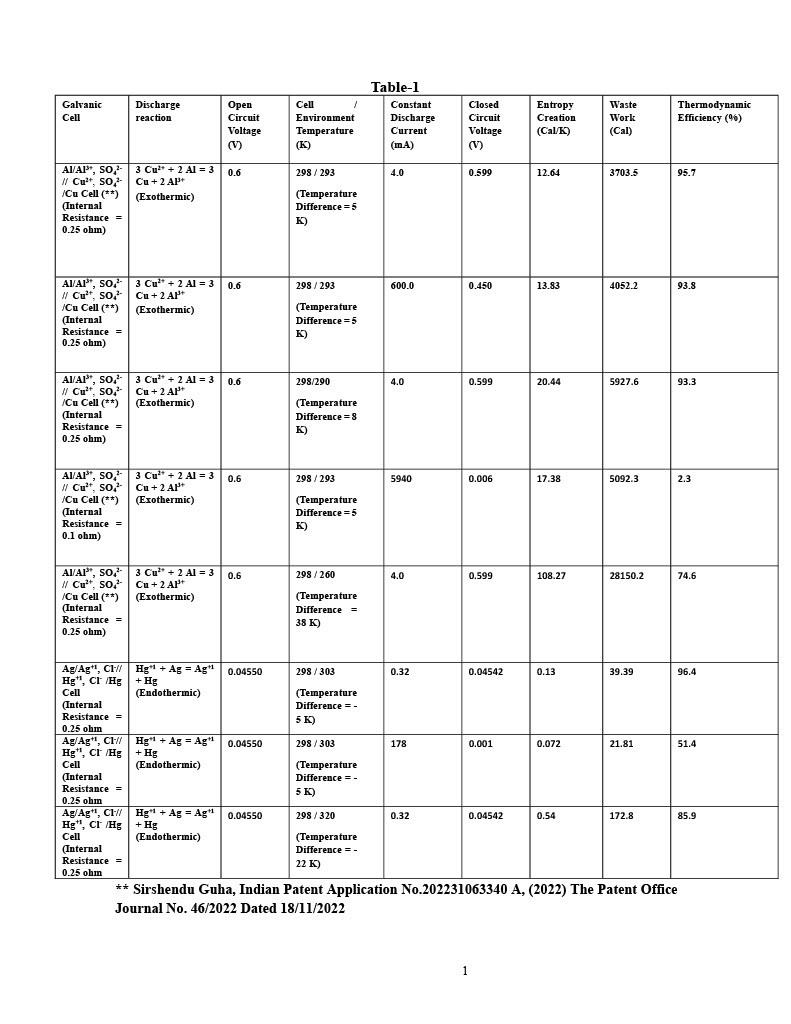
It is found that irreversible Entropy Creation, Waste Work and Thermodynamic Efficiency are all strongly dependent on environment temperature and discharge current.
Finally, for maximizing electrical energy output, a Galvanic Cell with exothermic discharge reaction should be operated at lower discharge current and at a cell operating temperature which is close to the environment temperature ensuring minimum difference between these two temperature values.
Similarly, a Galvanic Cell with endothermic discharge reaction should also be operated at lower operating current and at cell operating temperature which is close to the environment temperature maintaining minimum difference between these two temperatures for maximizing electrical energy output from the cell.
Lower cell discharge current and minimum difference between the cell and environment temperatures will ensure higher âavailability" which in turn will lead higher Thermodynamic Efficiency.
As an example, Table-1 is presented where some cell chemistries are shown which will establish the facts stated.