2023 AIChE Annual Meeting
(565g) Theoretical Analysis of Salt Selection for a Reverse Electrodialysis System
Authors
Arash Emdadi - Presenter, Fuji Film, Inc.
Jamie Hestekin, University of Arkansas
Lauren F. Greenlee, University of Arkansas
Bruce E. Logan, The Pennsylvania State University
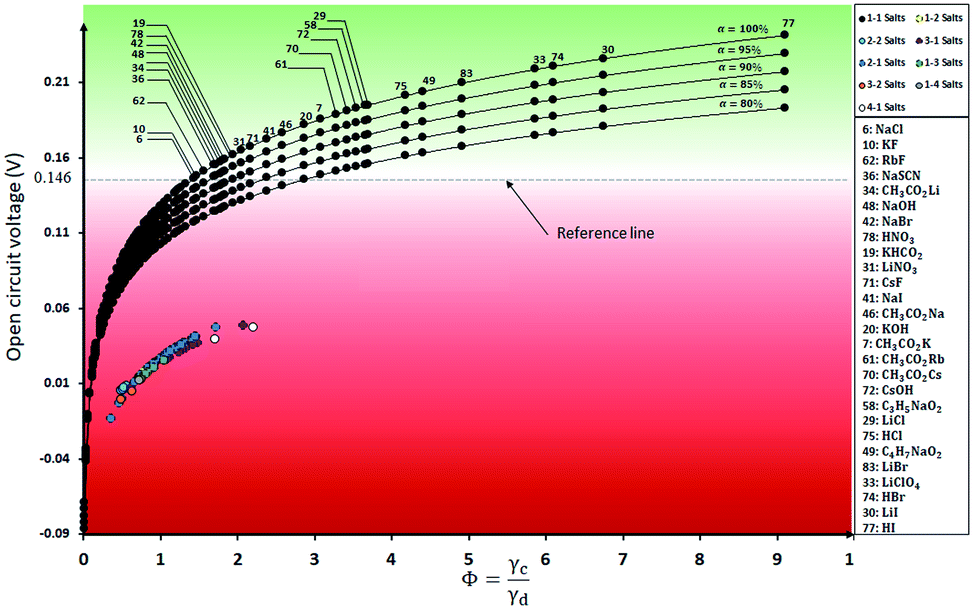
Reverse electrodialysis (RED) is a membrane technology used for electricity generation when two solutions with different salinity mix. The difference in the Gibbs free energy of concentrated and diluted solutions is the driving force for ion transport between channels and electricity generation. The type and nature of the components dissolved in the streams play an important role in electricity generation by RED. Parameters, including the valence of ions, the charge density of ions, and the activity coefficient of ions, affect the diffusion process between concentrated and diluted streams. A model was developed for screening salt solutions, including 174 monovalent and multivalent ions. In the first step, the theoretical open circuit voltage (OCVth) of 174 salt solutions, including mono and multivalent ions (AxBy; xâ{1,2,3,4}; yâ{1,2,3,4}) was calculated and compared with the reference salt (NaCl). The Bromley model was used for activity coefficients and OCVth calculation of the RED cell. The effect of ionic properties, including charge density, ionic radius, and hydration energy, on the OCVth was analyzed. In the second step, a model for hazard assessment was developed to select the salt solutions with minimum environmental, health, and physical hazards. Between the investigated salts, potassium formate (KHCO2), sodium bromide (NaBr), potassium acetate (KC2H3O2), and sodium acetate (NaC2H3O2) were found to be promising salts for RED applications due to high theoretical OCV, low hazard potential, and low cost compared to NaCl.