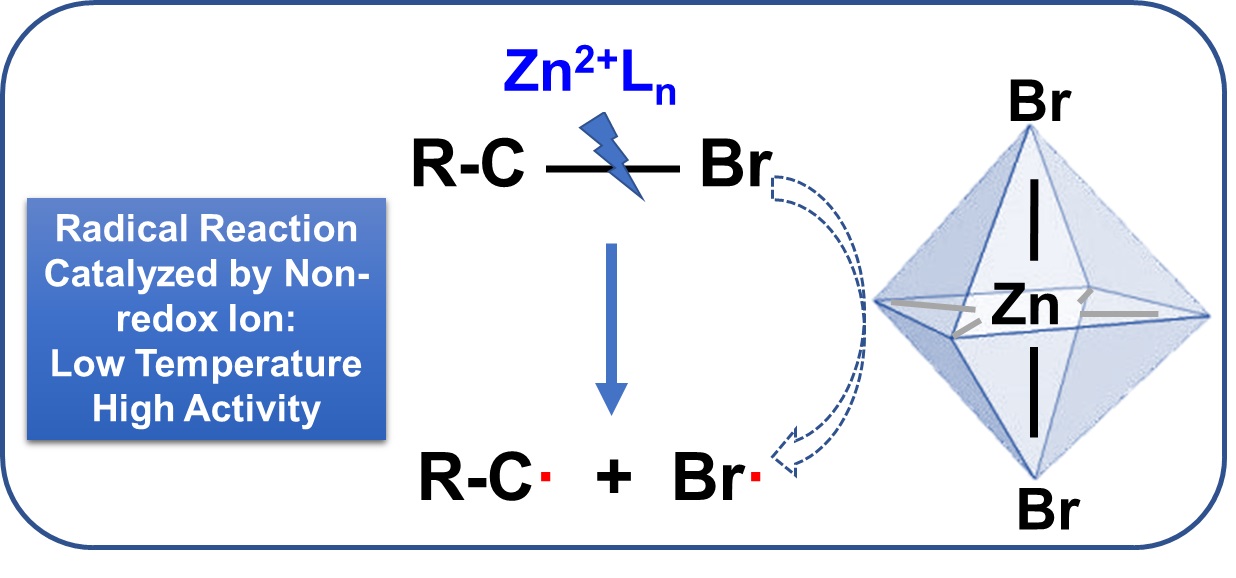
Facile separation of complex mixtures via catalytic reactions has gained interest in industrial manufacturing. Specifically, the Zn
2+ system has been employed to purify α-β-bromoethylbenzene mixtures by selectively decomposing α-bromoethylbenzene (α-B). We have confirmed that the non-redox metal ion Zn
2+ facilitates the homolysis of the CâBr bond in halohydrocarbons with benzyl bromide, which significantly promotes the generation of free radicals. While anionic complex intermediate formation has been reported as the primary mechanism for radical production from azobisisobutyronitrile (AIBN) and benzoyl peroxide (BPO) catalyzed by non-redox metal salts, which is not applicable to the Zn
2+ catalyzed decomposition of α-B.
In this study, we used AIBN/BPO homolysis mediated by non-redox metal ions as a reference to examine the unique mechanism of Zn2+-catalyzed α-B decomposition. Kinetic and thermodynamic analyses of various Zn2+/Cu2+ and AIBN/BPO/α-B combinations suggested that Zn2+-catalyzed α-B decomposition was not initiated by anion catalysis, contrary to previous reports. In situ Raman and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) revealed the activation and stretching of the C-Br bond in α-B when Zn2+ ions were present, while a radical scavenger experiment suggested a chain mechanism. Liquid-phase X-ray absorption fine structure (XAFS) and catalyst screening tests further confirmed that Zn2+-catalyzed α-B decomposition was a metal ion-dominated process leading to radical formation. We further extended the substrate scope to benzyl halides and allyl iodohydrocarbons and proposed this unprecedented mechanism which holds the potential to revolutionize fields such as polymerization.