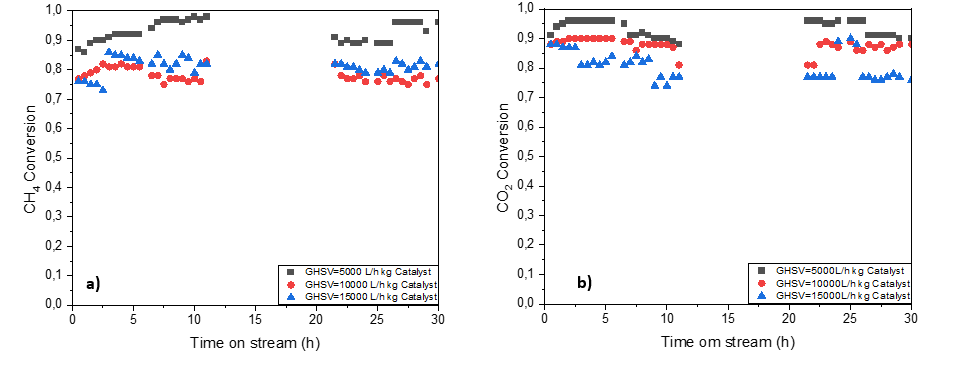
While emissions of greenhouse gases are increasing in the atmosphere due to human activities and industrial development which focuses mainly on fossil fuels as main source of energy. Efforts have been made to valorize these gases composed of carbon dioxide and methane to be used as reactants to produce syngas which is a mixture of CO and H
2 following the dry reforming reaction (DRM). However, one of the main challenges of DRM reaction is catalyst deactivation caused by coke deposition. The synthesis of a coke-resistant catalyst is a crucial step in DRM to ensure a good catalytic performance. Nickel-based catalysts are tested on Hydroxyapatite support. These catalysts were prepared by the wet impregnation method. The dry reforming reaction runs were carried out in a quartz fixed bed reactor at 800°C, atmospheric pressure, and different values of gas hourly space velocity (GHSV). CH
4 and CO
2 were emulsified with CH
4/CO
2 ratio equal to one. The results revealed high conversion reaching 93% for CH
4 and 98% for CO
2 at a low value of GHSV. Different characterization techniques were used to evaluate the physical and chemical properties of the fresh and spent catalysts such as: X-ray diffraction (XRD), electron microscope (SEM) coupled with energy-dispersive X-ray (EDX), temperature programmed reduction (TPR-H
2), thermogravimetric analysis (TGA) coupled with Gas chromatography mass spectrometry (GC-MS), Brunauer-Emmet-Teller (BET) and Barrett Joyner-Halenda (BJH) method. To sum up, Nickel based catalysts over hydroxyapatite showed a good performance and a good thermal stability. This later could be a key to minimize coke deposition which causes catalysts deactivation and eventually reduce the conversion of methane and carbone dioxide.