2023 AIChE Annual Meeting
(545f) Multiscale Simulations of Monoclonal Antibodies in High Concentration Formulations
Authors
Harold Hatch - Presenter, NIST
Christina Bergonzo, National Institute of Standards and Technology
Vincent K. Shen, National Institute of Standards and Technology
Violetta Burns Casamayor, Institute for Bioscience and Biotechnology Research
Guangcui Yuan, National Institute of Standards and Technology
Alexander V. Grishaev, Institute for Bioscience and Biotechnology Research
Yun Liu, National Institute of Standards and Technology
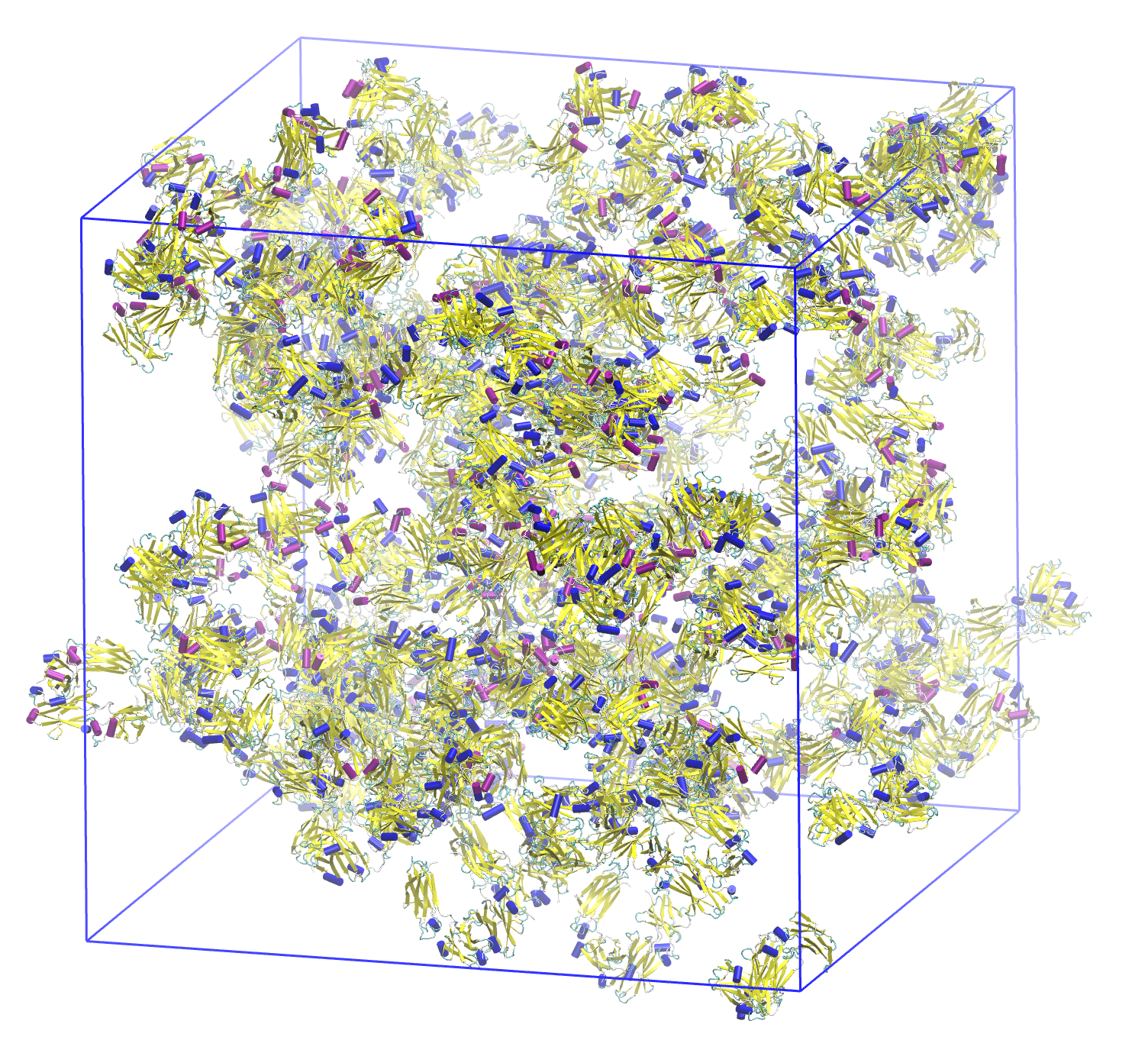
Although monoclonal antibodies (mAbs) are some of the most profitable and promising pharmaceuticals for targeted therapies, physical instabilities at high concentration including aggregation, high viscosity and phase separation cause problems for their manufacture, delivery to patients and long term stability. In this work, we present a multiscale methodology which uses all-atom modeling and experimental second osmotic virial coefficients to develop coarse-grained models for flat-histogram Monte Carlo simulations of hundreds of mAbs with sub-nanometer resolution. In this multiscale modeling approach, the major assumption is that mAb domains are held fixed so that their atomistic interactions with implicit solvent can be precomputed and therefore increase simulation efficiency by a few orders of magnitude. Although domains within the mAbs are rigid, the coarse-grained model includes the flexibility of the hinge region, which is often neglected but here shown to play an important role at mAb concentrations up to 150 g/L. This approach is also amenable to modeling excipients, co-formulations and surface interactions. Simulations were validated against experimental measurements and used to predict the physical stability of mAbs. These results highlight the potential for this multiscale approach to pre-screen pharmaceutical candidate mAbs in early stage development to avoid high concentration physical instabilities that can plague later stage development.