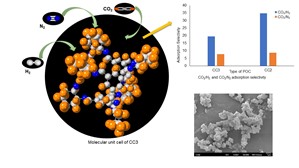
The selective capture of carbon dioxide over nitrogen and hydrogen is of great industrial interest in flue gas and hydrogen purification respectively. Microporous adsorbents are highly suitable materials to preferentially adsorb gases. In particular, Porous organic cages with tunable hierarchically ordered micropores, high surface area, and thermodynamic affinity for CO
2 make them appealing candidates for these applications. Herein, we demonstrate that two prototypical POCs denoted as CC3, and CC2 with limiting pore aperture of 3.6 can selectively separate CO
2 from N
2, and H
2. For CC3 adsorption selectivities as high as ~ 8 and ~20 for CO
2/N
2 and CO
2/H
2 respectively
were observed. For CC2 adsorption selectivities as high as ~ 9 and ~35 for CO
2/N
2 and CO
2/H
2 respectively
were observed. Interestingly, the adsorption selectivity of the studied gases correlated linearly with polarizability selectivity.