Palladium is an active catalyst for purifying alkene streams but often leads to over-hydrogenation and waste of raw ethylene due to the strong adsorption of ethylene on active sites. Various approaches have been explored to weaken the adsorption energy based on the electronic and geometric effects to enhance ethylene selectivity. However, due to the linear scaling relationships between adsorbates, the activation of acetylene is also severely restricted on palladium sites, resulting in decreased reactivity and high reaction temperature. Moreover, reducible metal oxides such as TiO
2, CeO
2, and GaCeO
x have been proposed for catalytic hydrogenation of alkynes with intrinsic alkenes selectivity. However, the hydrogenation activity is orders of magnitude lower than Pd-based catalysts due to the sluggish H
2 dissociation dynamics
. Considering the easy H
2 dissociation on palladium sites, it inspires us to decouple active sites for reactants activation to achieve efficient semi-hydrogenation of alkynes, where H
2 is activated on palladium sites and C
2H
2 is activated on reducible metal oxides such as TiO
x.
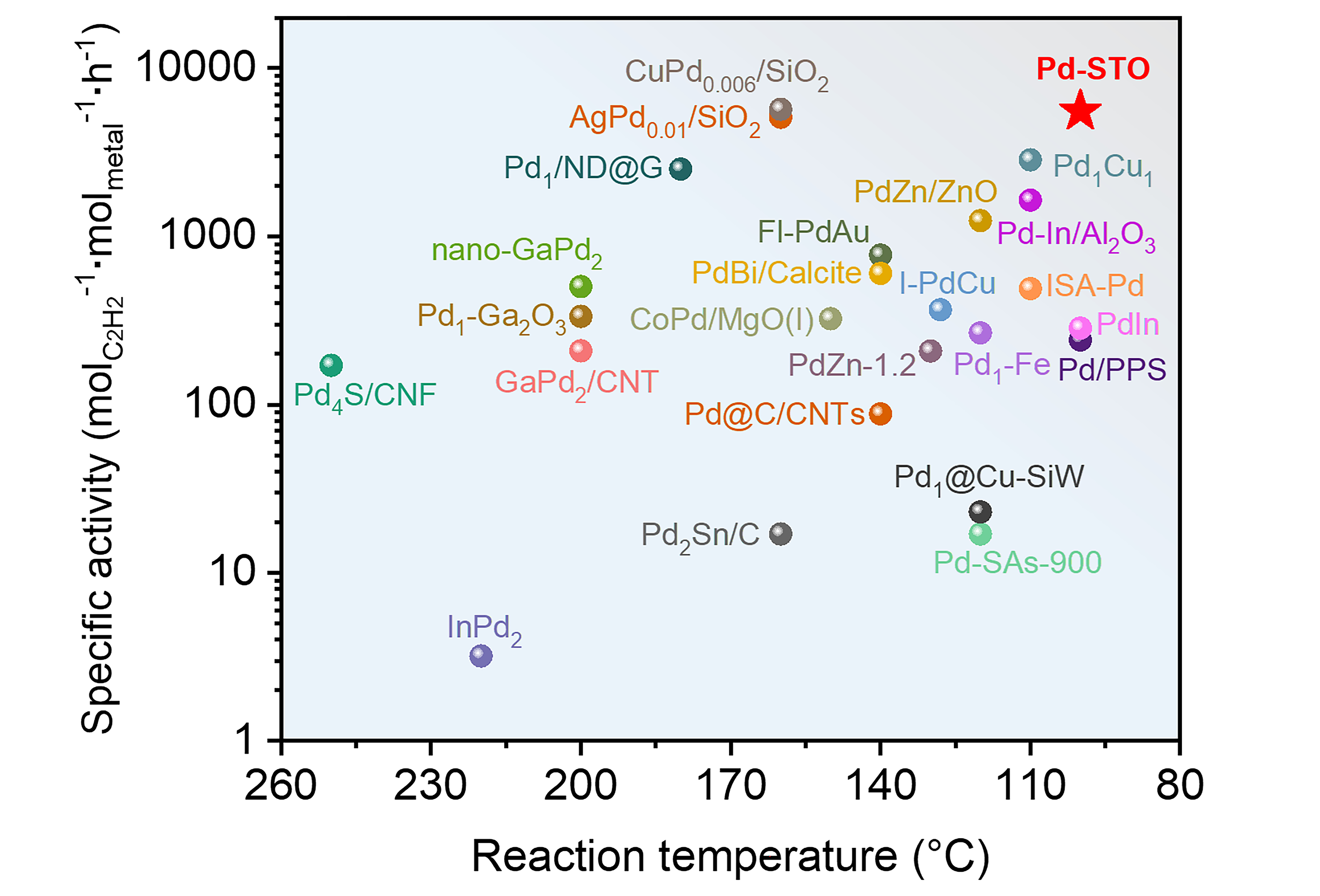
To this end, we fabricated a Pd-SrTiO3 (denoted as Pd-STO) catalyst with well-defined encapsulated Pd clusters using a doping-exsolution strategy based on SrTiO3 perovskite. SrTiO3 has been reported to exert an excellent ability to transport active hydrogen species at low-temperature. The Pd-STO catalyst takes advantage of the weaker adsorption of intermediate on the partially reduced oxide, TiOx, to enhance the ethylene selectivity. This catalyst shows good performance of 98% conversion and 92% selectivity with a specific activity of 5552 molC2H2-1·molmetal-1·h-1 at 100 â. The demonstrated catalyst design strategy paves an avenue for breaking the scaling relationship of hydrogenation catalysts by decoupling active sites for the activation of reactants.