2023 AIChE Annual Meeting
(467d) Experimental and Modeling Study of Lactose Crystallization: Development of a Chromatographic Method to Monitor Solution Composition
Accurate process understanding and optimization must take into account the isomerization of lactose. Hence, it is critical to have a robust and accurate measurement procedure to compute the correct driving force of the process, intended to be the supersaturation of alpha-lactose only. In addition, beta-lactose is known to influence the crystallization kinetics by acting as a nucleation and growth rate inhibitor [3] and, as the crystallization proceeds, it converts to alpha-lactose.
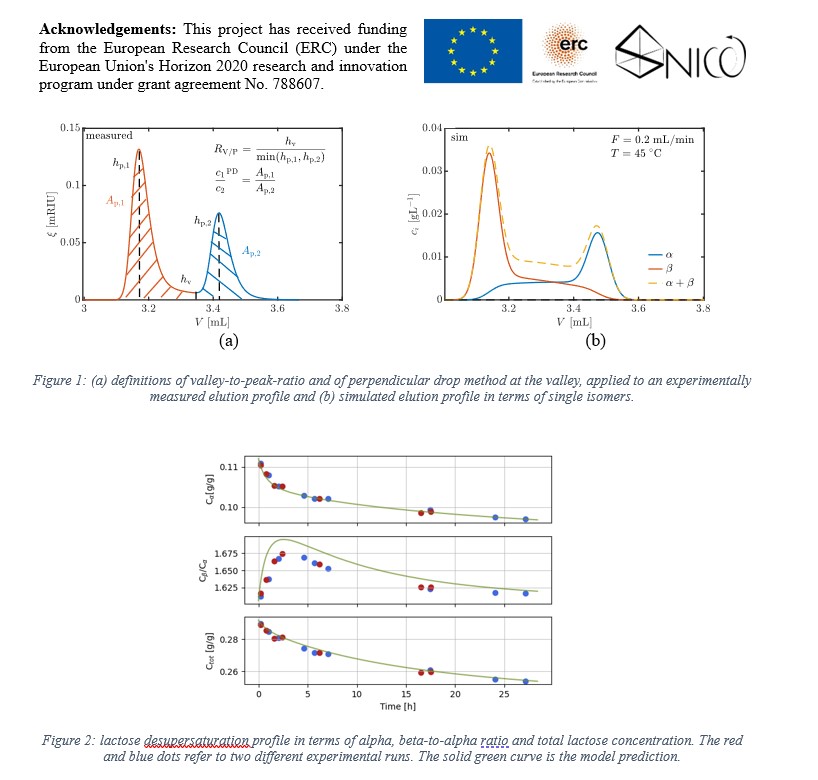
Therefore, the objective of this work is to establish a rigorous chromatographic protocol to quantify the composition of a mixture of reacting species. Indeed, the reaction introduces a non-gaussian band broadening that leads to progressive coalescence of the single unreacted peaks. Since it is impossible to reach baseline separation, analytical chromatography suffers from systematic errors during the subsequent quantification phase, that have been investigated via a model-based analysis. The measured elution profile, reported in Figure 1 (a), is post-processed using two different quantification strategies, that is, the perpendicular drop at the valley method and the curve fitting using the sum of two exponentially modified Gaussians (EMGs). Separation quality is assessed by the valley-to-peak ratio, that is, the ratio of the height of the valley that connects the peaks to the smallest of the two heights of the peaks. Computer simulations have been run using an in-house code that solves the equilibrium-dispersive-reaction model of chromatography for two solutes, alpha and beta, using a high-resolution finite-volume discretization scheme [4-5]. An example of simulated elution profile is reported in Figure 1 (b).
The chromatographic method has been employed to characterize the isomerization and crystallization kinetics of lactose within a rigorous population balance model, by tracking the evolution of alpha- and beta-lactose over time in seeded-batch desupersaturation experiments, as shown in Figure 2. The measurements have shown that, depending on the operating conditions, the crystallization rate could be faster than mutarotation rate and perturb the mutarotation equilibrium. Hence, the beta-to-alpha-ratio profile shows a maximum over time. State-of-the-art parameter estimation techniques have been used to evaluate different growth rate expressions by comparison with experimental data.
[1] I.K. Lappa, A. Papadaki, V. Kachrimanidou, A. Terpou, D. Koulougliotis, E. Eriotou, and N. Kopsahelis (2019): Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications, Foods 8, 347.
[2] F.J. Barba (2021): An Integrated Approach for the Valorization of Cheese Whey, Foods 10, 564.
[3] E. Simone, A.I.I. Tyler, D. Kuah, X. Bao, M.E. Ries, and D. Baker (2019): Optimal Design of Crystallization Processes for the Recovery of a Slow-Nucleating Sugar with a Complex Chemical Equilibrium In Aqueous Solution: The Case of Lactose, Org. Process Res. Dev. 23, 220â233.
[4] F. Ortner, H. Wiemeyer, M. Mazzotti (2017): Interconversion and chromatographic separation of carbohydrate stereoisomers on polystyrene-divinylbenzene resins, J. Chromatogr. A, 1517, 54-65.
[5] S. Trespi, M. Mazzotti (2023): manuscript in preparation.