Breadcrumb
- Home
- Publications
- Proceedings
- 2023 AIChE Annual Meeting
- Food, Pharmaceutical & Bioengineering Division
- Synthetic Biology and Microbiome in Agriculture, Food, and Health
- (387f) Understanding Gene Epistasis through Heterologous Systems

Thus, TIR1/AFBs are a family of signaling proteins that co-regulate auxin induced transcription, which consists of six members, TIR1 and AFB1-5. These proteins play a distinctive role in auxin signaling, whilst its full mechanisms are still unclear. Additionally, higher order SCF complexes are formed through the oligomerization of TIR1/AFBs and possibly play an essential role in the binding and degradation of Aux/IAAs. Studying the function of heterogeneous TIR1/AFB oligomers is critical to our understanding of how auxin signals are transduced to affect plant form and function. The specialized roles of these proteins have been identified, but the effects of hetero-dimerization on their concerted function has not been studied. To build up this concerted function and understand which genes interact we use a heterologous expression system in yeast as well as phenotypic assays using single and double mutants in Arabidopsis thaliana.
We investigated how pairs of TIR1/AFB auxin receptors interact to affect auxin-induced degradation of Aux/IAA17 transcriptional repressor. We found that heterodimerization of AFB1 with both TIR1 and AFB2 inhibits Aux/IAA17 degradation. We have also identified the oligomerization interface necessary for AFB1 to affect this inhibition. Furthermore, we examined the effect of this epistatic interaction in plant phenotype, and encountered significant effects of heterodimerization on the total number of lateral roots, primary roots growth, and primary root growth inhibition in response to exogenous auxin. Therefore, if we want to engineer plant function, it is critical to understand how large gene families act in concert.
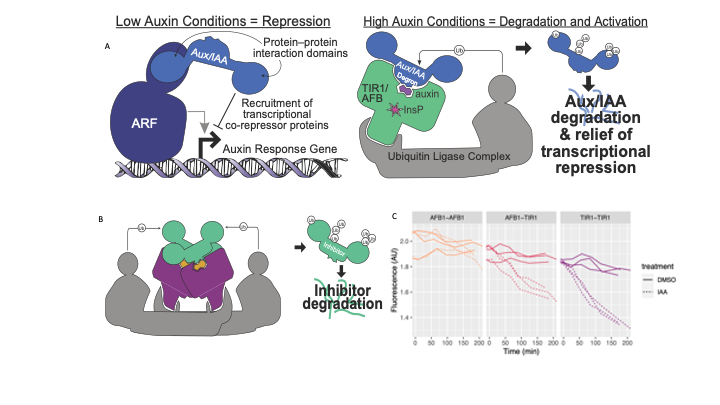
Figure 1. Auxin signaling pathway mechanism. A) In low auxin conditions Aux/IAA (blue) are bound to ARF (navy) inhibiting the response of auxin response genes. In high auxin condition, auxin acts as a bridge, bringing together Aux/IAA inhibitor and TIR1/AFBs receptors. These receptors ubiquitinate (Ub) the inhibitor targeting it for proteasomal degradation, and consequently relieving transcriptional repression. B) The complex interaction between auxin receptors that form dimers impacting how Aux/IAAs inhibitors are degraded. C) Shows the interaction between auxin receptors and mediated degradation of Aux/IAA17. We can see that AFB1-TIR1 interactions delay Aux/IAA17 degradation.