2023 AIChE Annual Meeting
(294b) Insights into Hydride Formation on Graphene-Supported Metal Nanostructures through a Combined Experimental and Theoretical Approach
Authors
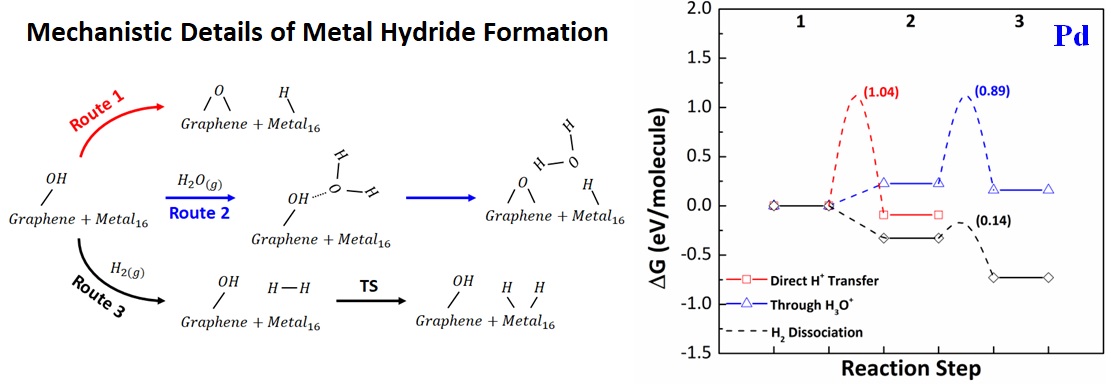
Vibrational density of states (VDOS) was collected through simulating AIMD trajectories to study the critical surface chemistry features, including O-H stretching region at 3200-4000 cm-1, presented in the experimental FT-IR spectra over the graphene oxide surface. An absence of hydride phase is also shown on Au cluster compared to Pd and Pt clusters from the XRD profiles. Thus, three mechanistic pathways were evaluated (in terms of DFT-based Gibbs free energy) for hydride formation on the surface of Au, Pd, and Pt clusters: 1) direct transfer of H+ from the surface OH group, 2) H3O+-mediated H+ transfer, and 3) transfer of H+ through H2 dissociation. We found that the hydride formation over supported metal clusters is thermodynamically and kinetically driven through the dissociation of H2 molecule on the metal surface, with Au being resistant to such reduction mechanisms. Overall, our results show that a different level of hydride formation and its effects on the catalytic activities is expected with graphene oxide surface decorated with various metals.