Cells of the adaptive immune system such as CD4
+ T cells, the innate immune system such as neutrophils, and hematopoietic stem cells (HSPCs), must transit to distal sites such as the lymph nodes, bone marrow, or sites of infection. The recruitment of t All cells of a hematopoietic lineage, such as CD4
+ T cells, neutrophils, monocytes/macrophages and even hematopoietic stem cells (HSPCs) must transit from the blood to distal sites such as the lymph nodes, bone marrow, or sites of infection. After initial braking and slow rolling, immune cells migrate along the vascular endothelium on a variety of adhesion ligands, the most prominent being Intracellular Adhesion Molecule-1 (ICAM-1), to find transmigration sites. Previous work by us and others has demonstrated an interesting phenomenon where cells such as murine and human T lymphocytes (1, 2), HSPCs (3), and marginal zone B-cells can migrate efficiently upstream (against the direction of shear-flow) on ICAM-1 surfaces and endothelial monolayers through Lymphocyte Functional Antigen-1 (LFA-1) interactions. Recent work of ours has furthered the phenomenon of upstream migration by demonstrating that innate immune cells such as neutrophils, which express a second ICAM-1 binding integrin named Macrophage-1 antigen (Mac-1), can migrate upstream when Mac-1 is blocked with antibodies (4). To that end, monocytes and macrophages also express both LFA-1 and Mac-1 and their ability to migrate against the direction of shear flow remains undetermined. To see if the loss of Mac-1 expression could induce the upstream migration behavior, we utilized blocking antibodies to neutralize and genetic engineering to silence Mac-1 on the human macrophage cell line U937, the mouse macrophage cell line Raw264.7, and human peripheral blood monocytes. M0 macrophages of all cell types were also polarized into M1 or M2 macrophages to determine the effect of polarization on the propensity to crawl upstream.
Flow cytometry was performed to determine the integrin expression profiles of primary PBMCs, U937, and the raw264.7 cell lines for both the control and genetically engineered lines. Raw 246.7 cells were already in M0 while U937 cells were stimulated with 100ng/ml PMA for 48hrs and PBMCs were stimulated with 2ng/ml M-CSF for 7 days to reach M0. All cells were subsequently polarized with 20ng/ml IFN-γ and 100ng/ml LPS for M1 or 20ng/ml IL-4 for M2. Cells were then screened for migratory behavior at static (0s-1), low (100s-1, 200s-1), and high (400s-1,800s-1) shear rates after settling on slides coated with either ICAM-1 at a fixed mass ratio of 5μg/ml. Migration index, number of migrating cells (cells/mm2), persistence time (min), speed (µm/min), motility coefficient (µm2/min) and percentage of cells migrating upstream (%) were calculated using a combination of Image J tracking and Matlab analysis.
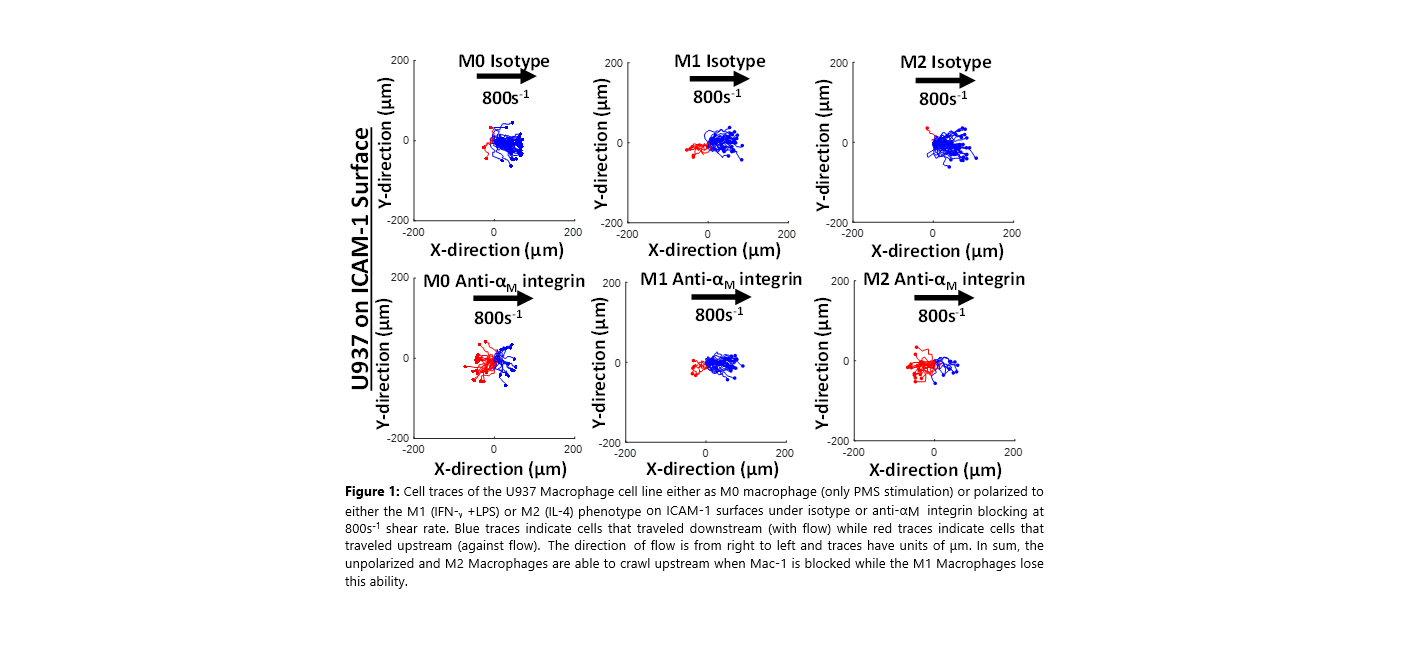
To this end, we demonstrate that the M0 human macrophage line U937, M0 murine macrophage line raw264.7, and human PBMCs differentiated into M0 macrophages, all of which express a second ICAM-1 ligand Macrophage-1 antigen (Mac-1), can migrate upstream on ICAM-1 surfaces and endothelial layers when Mac-1 is blocked with antibodies or genetically engineered with shRNA. Furthermore, we demonstrate when U937, Raw 264.7, or PBMCs are polarized into M2 macrophages, they retain the ability to migrate upstream on ICAM-1 when Mac-1 binding is disrupted (Figure 1). On the other hand, when the same cell lines and primary macrophages are polarized into the M1 phenotype, they lose the ability to migrate upstream on ICAM-1 surfaces when Mac-1 binding is disrupted. Here we demonstrate for the first time that macrophages can be induced to crawl against the direction of shear flow and that manipulating Mac-1 expression is necessary to achieve upstream migration. Furthermore, only M0 and M2 macrophages are susceptible to Mac-1 perturbation as M1 macrophages still crawl downstream even with the loss of Mac-1 expression.
This has direct implications in understanding of how macrophages traffic to distal sites and tissues also holds therapeutic potential in controlling the ability of macrophages upstream. Furthermore, it explores the unique and distinct modes of motility that anti-inflammatory (M2) macrophages use in comparison to pro-inflammatory (M1) macrophages to traffic to the inflammatory site.
References:
- M. P. Valignat et. al, Biophys J., 2013, 104(2):322-331.
- G.A. Dominguez, N.R. Anderson, and D.A. Hammer, Integr Biol., 2015, 7(3):345-355.
- A. Buffone Jr., N.R. Anderson, and D.A. Hammer, J Cell Sci., 2018, 131(1): jcs205575
- A Buffone Jr, N. R. Anderson, and D.A. Hammer, Biophys J, 2019, 117(8):1393-404.
Acknowledgements: This work was funded by NIGMS GM143357-R01 TO ABJ.