Carbon dioxide (CO
2) is the largest contributor among the global warming gases and is responsible for increasing temperatures through a process called the greenhouse effect. One way to mitigate CO
2 is to convert CO
2 electrochemically into value-added chemicals such as C1 (carbon monoxide, formate/formic acid) and C
2+ products. Amongst these technologies, electrochemical reduction of CO
2 to CO is most mature technology in laboratory. Stable performance of CO
2 electrolysis to CO and O
2 for up to 4000hrs with negligible degradation has been demonstrated when operating with pure CO
2 1,2. However, industrial CO
2 sources usually contain other species such as nitrogen (N
2), Oxygen (O
2), nitrogen oxides (NO
x), volatile sulfur compounds (VSC: SO
2, H
2S, COS), volatile oxygenates (VO
X: ethanol) and volatile hydrocarbons (VHC). In this work, we investigate the effects of H
2S up to 25ppm in CO
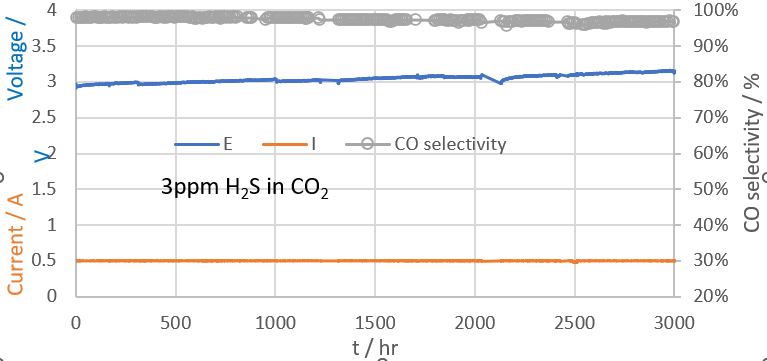
2 on the performance of anion exchange membrane CO
2 electrolyzers (AEMCEs). Figure 1 shows the stable performance of AEMCE for up to 3000hr. We will discuss the possible degradation mechanisms related to H
2S.
Figure 1 Long term stability of CO2 electrolyzer running at 500mA (100mA/cm2) in the presence of 3ppm of H2S in CO2 stream