Chemical recycling of plastics via âconversionâ refers to the use of chemical transformations to convert plastic waste into an oil-like hydrocarbon stream. In conversion schemes via thermal pyrolysis the product oil typically has a high olefins content (~50 wt%) [1,2,3]. Further processing of this pyrolysis oil in the refining/petrochemical industry, especially when coâfeeding pyrolysis oil with fossil streams, requires a previous hydrodenitrogenation step to guarantee product quality and protect catalyst in downstream hydrocracking processes [4,5,6].
To acquire a deeper understanding of the combined hydrodenitrogenation-olefin saturation, an experimental investigation of the hydrotreating of a mixture containing hexadecene, octadecene and quinoline has been performed. A commercially available NiMoP/Al2O3 catalyst was employed in a three-phase Robinson Mahoney reactor at a H2/HC ratio of 890 Nm3/m3 and total pressure of 90 bar while the temperature ranged between 300 and 350 °C and the LHSV varied between 0.4 and 1 h-1.
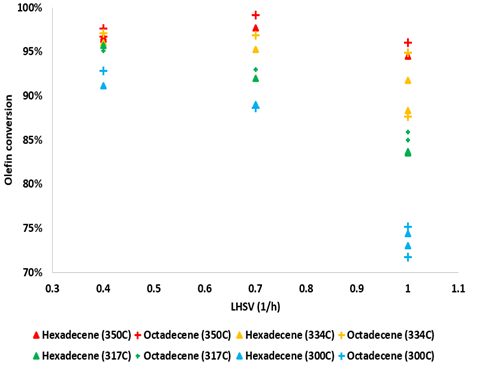
In general, as shown in Figure 1, it was observed that olefin conversion decreased dramatically with LHSV at 300 °C being the lowest between 70% and 75% at the highest LHSV. The higher the temperature the less significant the impact of the LHSV on the conversion was, given the high conversion that was achieved, i.e., at 350 °C, the olefin conversion always exceeded 95%. Quinoline conversion was always higher than 95%, the maximum yield of denitrogenated products amounting to 15% only. The hydrogenated intermediate 1,2,3,4 tetrahydroquinoline (14THQ) was identified as the major product in all cases.
Figure 1. Olefins conversion vs. LHSV (h-1) at different operating temperatures and 90 Bar
By comparing these results with similar studies found in literature it can be deduced that olefin saturation interferes with hydrogenation and ring opening reactions of the intermediates of quinoline hydrodenitrogenation as lower nitrogen conversions than in the literature at similar operating conditions were obtained [7,8,9,10,11].
As a future work intrinsic kinetic parameters for olefin hydrogenation obtained from this experimental campaign are intended to be used to tune olefins hydrogenation kinetics in an industrial scale reactor model for hydroprocessing (hydrotreating + hydrocracking) configurations where the reactor is co-fed with a fossil-pyrolysis oil mixture [12].
Keywords: chemical recycling, pyrolysis oil, olefin saturation, quinoline hydrodenitrogenation, hydrotreating
References
[1] D. Almeida and M. de F. Marques, âThermal and catalytic pyrolysis of plastic waste,â PolÃmeros, vol. 26, pp. 44â51, Mar. 2016, doi: 10.1590/0104-1428.2100.
[2] M. Kusenberg et al., âA comprehensive experimental investigation of plastic waste pyrolysis oil quality and its dependence on the plastic waste composition,â Fuel Processing Technology, vol. 227, p. 107090, Mar. 2022, doi: 10.1016/j.fuproc.2021.107090.
[3] H. Dao Thi, M. R. Djokic, and K. M. Van Geem, âDetailed Group-Type Characterization of Plastic-Waste Pyrolysis Oils: By Comprehensive Two-Dimensional Gas Chromatography Including Linear, Branched, and Di-Olefins,â Separations, vol. 8, no. 7, Art. no. 7, Jul. 2021, doi: 10.3390/separations8070103.
[4] C. S. Raghuveer, J. W. Thybaut, R. De Bruycker, K. Metaxas, T. Bera, and G. B. Marin, âPyridine hydrodenitrogenation over industrial NiMo/γ-Al2O3 catalyst: Application of gas phase kinetic models to liquid phase reactions,â Fuel, vol. 125, pp. 206â218, Jun. 2014, doi: 10.1016/j.fuel.2014.02.017.
[5] Q. Xin, A. Alvarez-Majmutov, H. D. Dettman, and J. Chen, âHydrogenation of Olefins in Bitumen-Derived Naphtha over a Commercial Hydrotreating Catalyst,â Energy Fuels, vol. 32, no. 5, pp. 6167â6175, May 2018, doi: 10.1021/acs.energyfuels.8b00344.
[6] Q. Xin, A. Alvarez-Majmutov, R. Gieleciak, J. Chen, and H. Dettman, âHydrotreating of Olefins in Thermally Processed Bitumen under Mild Conditions,â Energy & Fuels, vol. 33, Mar. 2019, doi: 10.1021/acs.energyfuels.9b00157.
[7] âHydrodenitrogenation of quinoline and its intermediates over sulfided NiW/γâAl2O3 in the absence and presence of H2S - Luan - 2009 - Asia-Pacific Journal of Chemical Engineering - Wiley Online Library.â https://onlinelibrary.wiley.com/doi/10.1002/apj.322 (accessed Mar. 31, 2022).
[8] C. Tu, S. Liu, C. Liu, X. Wu, Q. Chen, and W. Huang, âSingle-Pot Synthesis of NiMo-Al2O3 Catalyst for Quinoline Hydrodenitrogenation,â Energy Fuels, vol. 35, no. 2, pp. 1120â1128, Jan. 2021, doi: 10.1021/acs.energyfuels.0c03186.
[9] S. Tian, X. Li, A. Wang, Y. Chen, H. Li, and Y. Hu, âHydrodenitrogenation of Quinoline and Decahydroquinoline Over a Surface Nickel Phosphosulfide Phase,â Catal Lett, vol. 148, no. 6, pp. 1579â1588, Jun. 2018, doi: 10.1007/s10562-018-2370-z.
[10] M.-T. Nguyen, M. Tayakout-Fayolle, G. D. Pirngruber, F. Chainet, and C. Geantet, âKinetic Modeling of Quinoline Hydrodenitrogenation over a NiMo(P)/Al2O3 Catalyst in a Batch Reactor,â Ind. Eng. Chem. Res., vol. 54, no. 38, pp. 9278â9288, Sep. 2015, doi: 10.1021/acs.iecr.5b02175.
[11] B. Mattson et al., âHeterogeneous Catalysis: The Horiuti-Polanyi Mechanism and Alkene Hydrogenation,â Journal of Chemical Education, vol. 90, pp. 613â619, May 2013, doi: 10.1021/ed300437k.
[12] Pernalete C., Ibanez J., Van Geem K., Thybaut J., "Hydrocracking of complex mixtures: From bulk properties, over fundamental kinetics to detailed product composition," Catalysis Today, vol. 378 (2021) pp. 189â201. doi: 10.1016/j.cattod.2021.06.010.