Capture of CO
2 is a critical component in producing valuable reuse products from fossil fuel-based processes. It is generally accepted that current commercial technologies for capturing CO
2 are too energy intensive and thus cost prohibitive for implementation on coal based power plants. Hence, there is a critical need for development of new materials that can capture CO
2 reversibly with acceptable energy and cost performance for these applications. Accordingly, solid sorbents have been reported in several previous studies to be promising candidates for CO
2 sorbent applications through a reversible chemical transformation due to their high CO
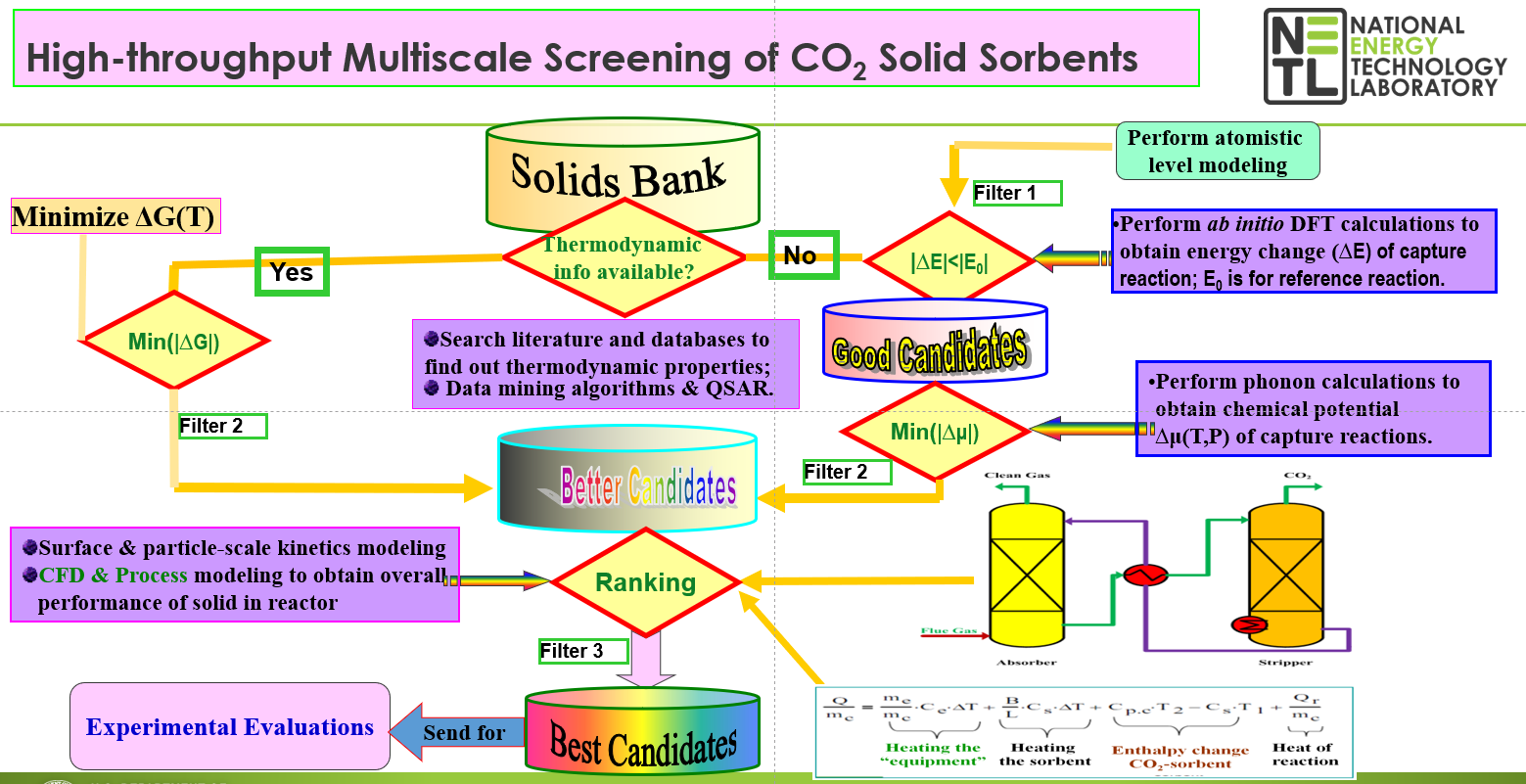
2 absorption capacities at moderate working temperatures. By combining thermodynamic database mining with first principles density functional theory and phonon lattice dynamics calculations, a theoretical screening methodology to identify the most promising CO
2 sorbent candidates from the vast array of possible solid materials have been proposed and validated at the National Energy Technology Laboratory (NETL). The advantage of this method is that it identifies the thermodynamic properties of the CO
2 capture reaction as a function of temperature and pressure without any experimental input beyond crystallographic structural information of the solid phases involved. The calculated thermodynamic properties of different classes of solid materials versus temperature and pressure changes were further used to evaluate the equilibrium properties for the CO
2 adsorption/desorption cycles. Per the requirements imposed by the pre- and post- combustion technologies and based on our calculated thermodynamic properties for the CO
2 capture reactions by the solids of interest, we are able to identify only those solid materials for which lower capture energy costs are expected at the desired pressure and temperature conditions. These CO
2 sorbent candidates were further considered for experimental validations.
However, at a given CO2 pressure, the turnover temperature (Tt) of an individual solid capture CO2 reaction is fixed and may be outside the operating temperature range (ÎTo) for a particularly capture technology. In order to shift such Tt for a solid into the range of ÎTo, its corresponding thermodynamic property must be changed by changing its molecular structure by mixing with other materials or doping with other elements. Our results demonstrate that by mixing or doping two or more materials to form a new material, it is possible to synthesize new CO2 sorbents which can fit the needs for industrial applications.
Recent experimental and calculated results show that some solid sorbents can serve as bi-functional materials: CO2 sorbent and CO oxidation catalyst. Such dual functionality could be used for removing both CO and CO2 after water-gas-shift to obtain H2 with high purity.