2023 AIChE Annual Meeting
(156f) Molecular and Process Modelling of Selected Novel Solvents for CO2 Capture
Authors
Given the recent advancement in molecular modeling tools, molecular-based equations of state provide a suitable platform for the design of modelling screening frameworks. To better enable the implementation of alternative amines for CO2 capture, a comprehensive understanding of their properties is essential. The main objective of this work is to develop a robust solvent screening tool using the soft-SAFT molecular-based equation of state (EoS) to obtain relevant thermodynamic properties for assessing novel solvents for CO2 capture. A collection of 40 alternative novel amines were included in this work, belonging to six families: (1) aminoalcohols, (2) alkylethanolamines, (3) dialkylaminoalcohols, (4) cyclic amines, (5) diamines, and (6) multiamines. The soft-SAFT molecular models of these amines were developed to calculate the thermophysical properties of pure amines such as density, vapor pressure, heat capacity and the heat of vaporization. Soft-SAFT also allows the calculation of other thermodynamic properties such as viscosity and surface tension by coupling the equation with appropriate theories, such as free volume theory for viscosity and density gradient theory for surface tension. The capability of soft-SAFT to describe the phase behaviour is a determining factor for the optimization of absorption/desorption process for CO2 capture. Alongside properties for the aqueous mixtures of amines inclusive of density, vapor-liquid equilibrium and viscosity, we calculated the CO2 absorption capacities at relevant process conditions.
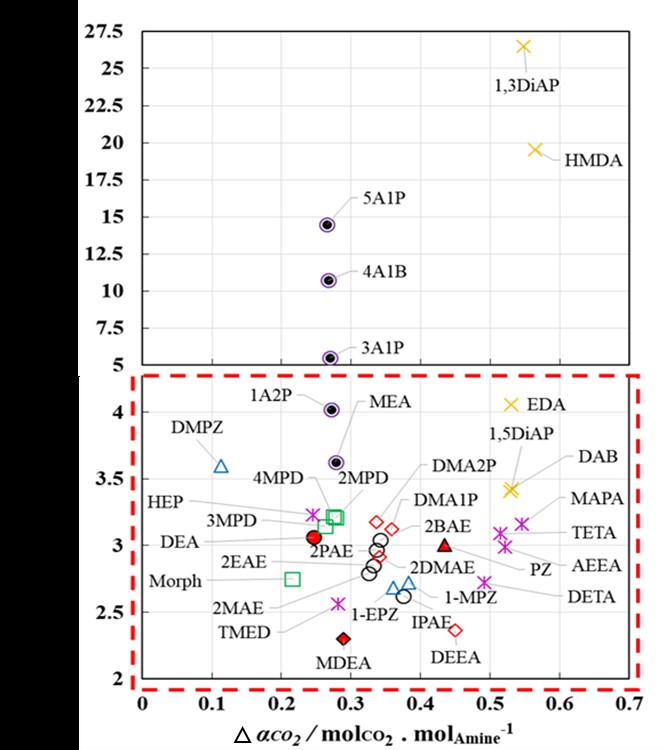
This work highlights the predictive power of soft-SAFT EoS in cases where no experimental data was available. The modeling was extended to calculate two key performance indicators, namely, cyclic capacity (Îα) which is the amount of CO2 effectively separated from the capture plant and the regeneration energy (Qregen) which is linked to the reboiler duty of the stripper column and is the energy required to desorb the CO2 from the rich amine stream, as screening criteria to determine the most optimal solvent. In our search for the most efficient replacement amine to the golden standard MEA, both 2-Diethylaminoethanol (DEEA) and Diethylenetriamine (DETA) showed promising potential in terms of a high cyclic capacity (Îα) and low regeneration energy (Qregen), shown from Figure-1. Results presented here bolster the significance of using Soft-SAFT molecular equation of state as a practical tool in the search for novel amines for CO2 capture.
For a holistic analysis of carbon capture, the impact of aqueous viscosity on the mass transfer coefficient and the reaction kinetics on the reaction rate of CO2 solubility are two important parameters that can be further studied at the target process conditions of 313K for 30 wt% amine. Moreover, the integration of these thermodynamic models with detailed process modeling such as ASPEN HYSYS to examine the techno-economic feasibility can further aid the search for novel solvents by providing a strong base to calculate and predict relevant thermodynamic properties.
Figure-1 Caption: Scatter plot of the Regeneration energy (Qregen) with cyclic capacity (Îα) of 36 novel amines.