2023 AIChE Annual Meeting
(152w) Low-Cost, Made-in-India Hollow Fiber Membranes (HFMs) for Hemodialysis Application
From the Indian point of view, currently, most of the hemodialysis instruments, accessories, and consumables are imported, including the HFM dialyzer.
So, if we start manufacturing it here itself, it will solve the problem to provide dialysis facility to large population suffering from kidney failure. We are working towards the development of biocompatible HFMs in large scale to deploy it for hemodialysis application. Our HFMs has salient features like; novel composition, low cost, excellent hemocompatibility, high uremic toxins removal efficiency, high permeation flux, minimal side-reactions [2]. Also, we are working closely with the industry with scaled up the manufacturing process, potting, housing and fabrication of dialyzers.
This work is in the phase of animal trial and our aim is to deploy our dialyzer for veterinary use. We will go for large animal models in future and subsequently for human use to benefit the needy.
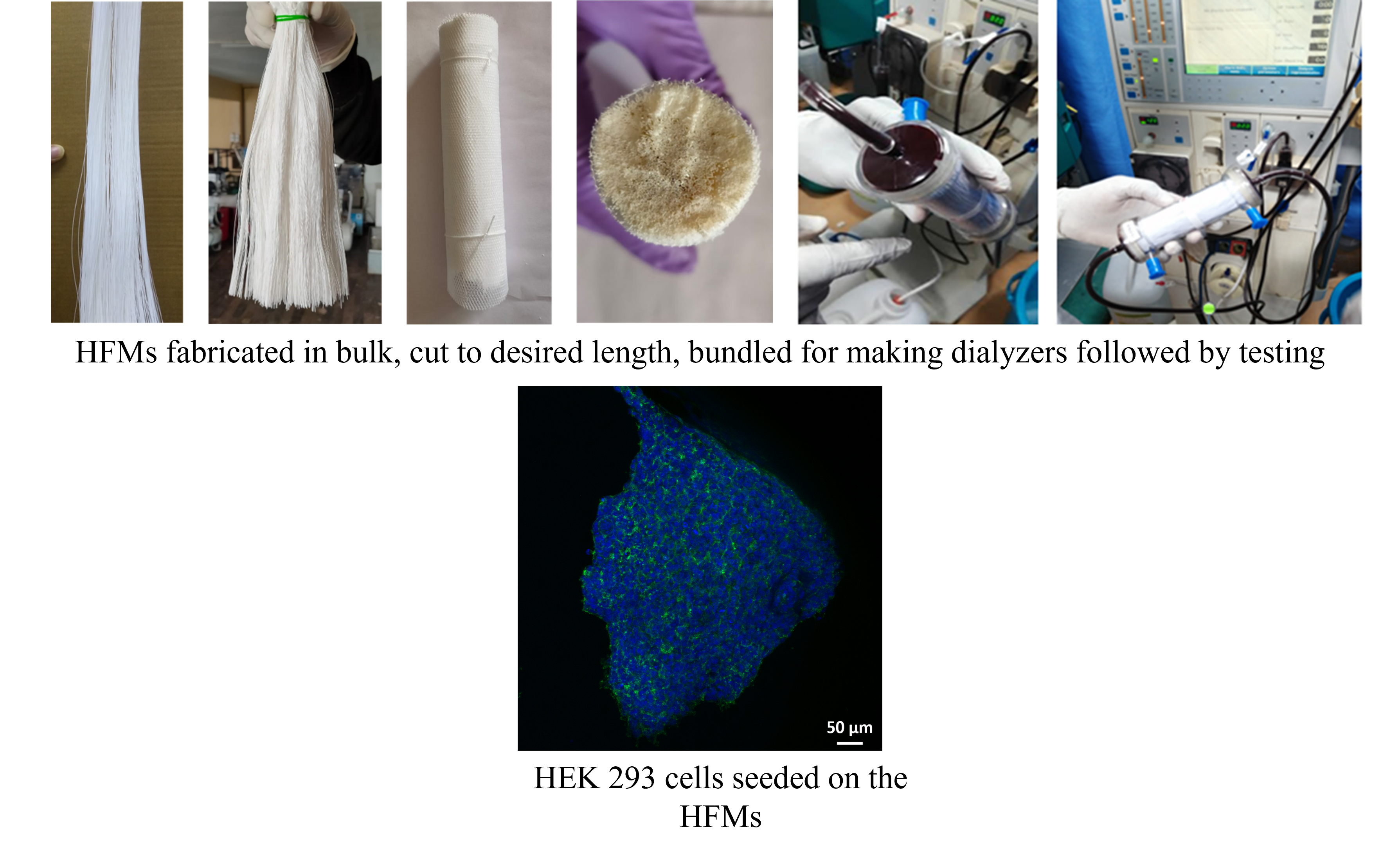
We also utilize these HFMs for bioartificial kidney application with kidney cells seeded on the HFMs to perform dual functions: mimic the metabolic functions of natural kidney along with detoxification. This can be used extracorporeally and can revolutionize the human healthcare significantly when extensive research is done in this field.
We have developed biocompatible polymeric material based HFMs for BAK application. This modified biocompatible HFMs showed improved hydrophilicity and cytocompatibility, allowing growth and proliferation of HEK-293 cells on the HFMs. The confocal images of the HFMs seeded with kidney cells showed cells were gathered to form spheroids type structure. The ultrafiltration coefficient of HFMs was also high 140.885 ±10 ml/m2/h/mmHg which shows the enhanced separation performance of the modified HFMs. The membranes also showed better hemocompatibility (< 5% hemolysis limit) and low value of complement activation i.e., SC5b9 marker level (10.71ng/ml). Thus, the above results corroborate that our biocompatible polymer is suitable for BAK application.
Keywords: Hollow fiber membranes; hemodialysis; bioartificial kidney; biomaterial; hemocompatibility; biocompatible
Acknowledgement: WRCB IIT Bombay, Dr. Akshay Modi, Dr. Surendra Kumar Verma, Vikash Kumar Gupta
References
[1] S. K. Agarwal and R. K. Srivastava, âChronic kidney disease in India: Challenges and solutions,â Nephron - Clin. Pract., vol. 111, no. 3, pp. 197â203, 2009, doi: 10.1159/000199460.
[2] G. J. Dahe, R. S. Teotia, S. S. Kadam, and J. R. Bellare, âThe biocompatibility and separationperformance of antioxidative polysulfone/vitamin E TPGS composite hollow fiber membranes,â Biomaterials, vol. 32, no. 2, pp. 352â365, 2011, doi:10.1016/j.biomaterials.2010.09.005.