2023 AIChE Annual Meeting
(146d) Eutectic Electrolytes for Secondary Al-CO2 Batteries
Authors
Cameron Carugati, University of New Mexico
Madalin Alderete, University of New Mexico
Shuya Wei, University of New Mexico
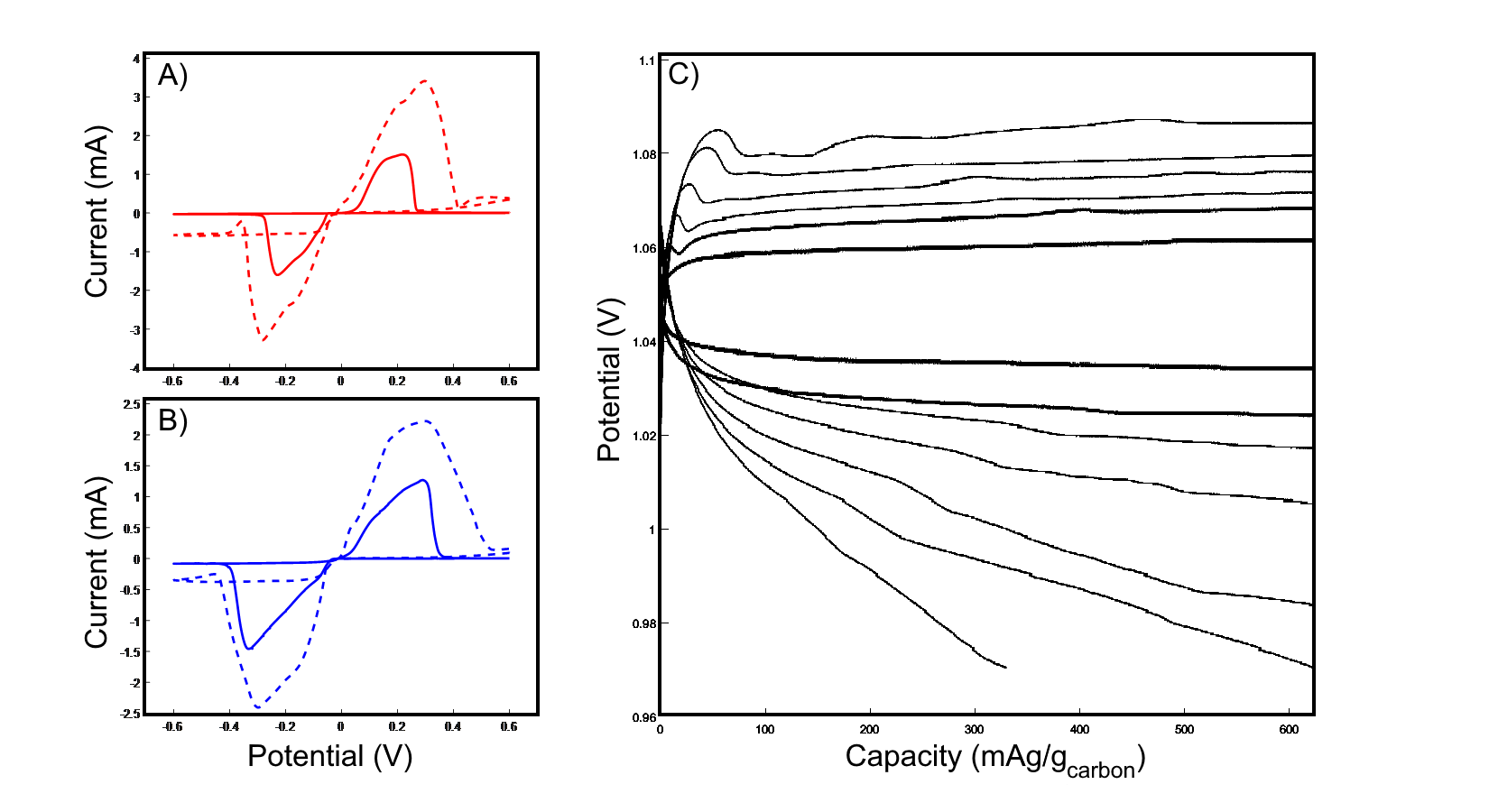
Metal-CO2 batteries have emerged as a promising strategy for enhancing energy storage technology while capturing/concentrating carbon dioxide. We previously demonstrated an Al-CO2 battery utilizing a homogeneous iodine-based redox mediator to enable the reversible discharge and charge of the battery with an ultra-low overpotential of 0.05V (Fetrow et al., ACS Appl. Mater. Interfaces 2023, 15, 10, 12908â12914). The imidazole-based electrolyte employed earlier is common for aluminum stripping/plating but is expensive, corrosive, and hygroscopic. In this study, we investigate several alternative electrolytes. These include AlCl3-based melts previously explored for aluminum-ion batteries, as well as novel AlI3-based eutectic melts. As shown in Figure 1, both urea- and acetamide-AlCl3 melts are compatible with the aluminum iodide homogeneous catalyst. We characterized electrolyte performance electrochemically and investigated the mechanisms enabling ionic liquid formation and performance using Raman and NMR spectroscopies. These findings facilitate the development of a practical Al-CO2 battery.
Figure 1: Cyclic voltammetry and galvanostatic discharge studies of alternative ionic liquids for Al-CO2 batteries. A, B) Cyclic voltammogram of coin cells with aluminum and stainless steel electrodes using 1.3:1 AlCl3:acetamide and 1.3:1 AlCl3:urea electrolytes, respectively. Dashed lines indicate cells without added 0.05M AlI3. C) Galvanostatic discharge of an Al-CO2 battery with aluminum and nanostructured carbon cathodes using a 1.3:1 AlCl3:acetamide electrolyte containing 0.05M AlI3. Discharged at 20 mA/gcarbon to a capacity of 620 mAh/gcarbon.