2023 AIChE Annual Meeting
(10e) Immunobioengineering of a Three-Dimensional Polymeric Scaffold-Based Implantable Thymic Organoid
Authors
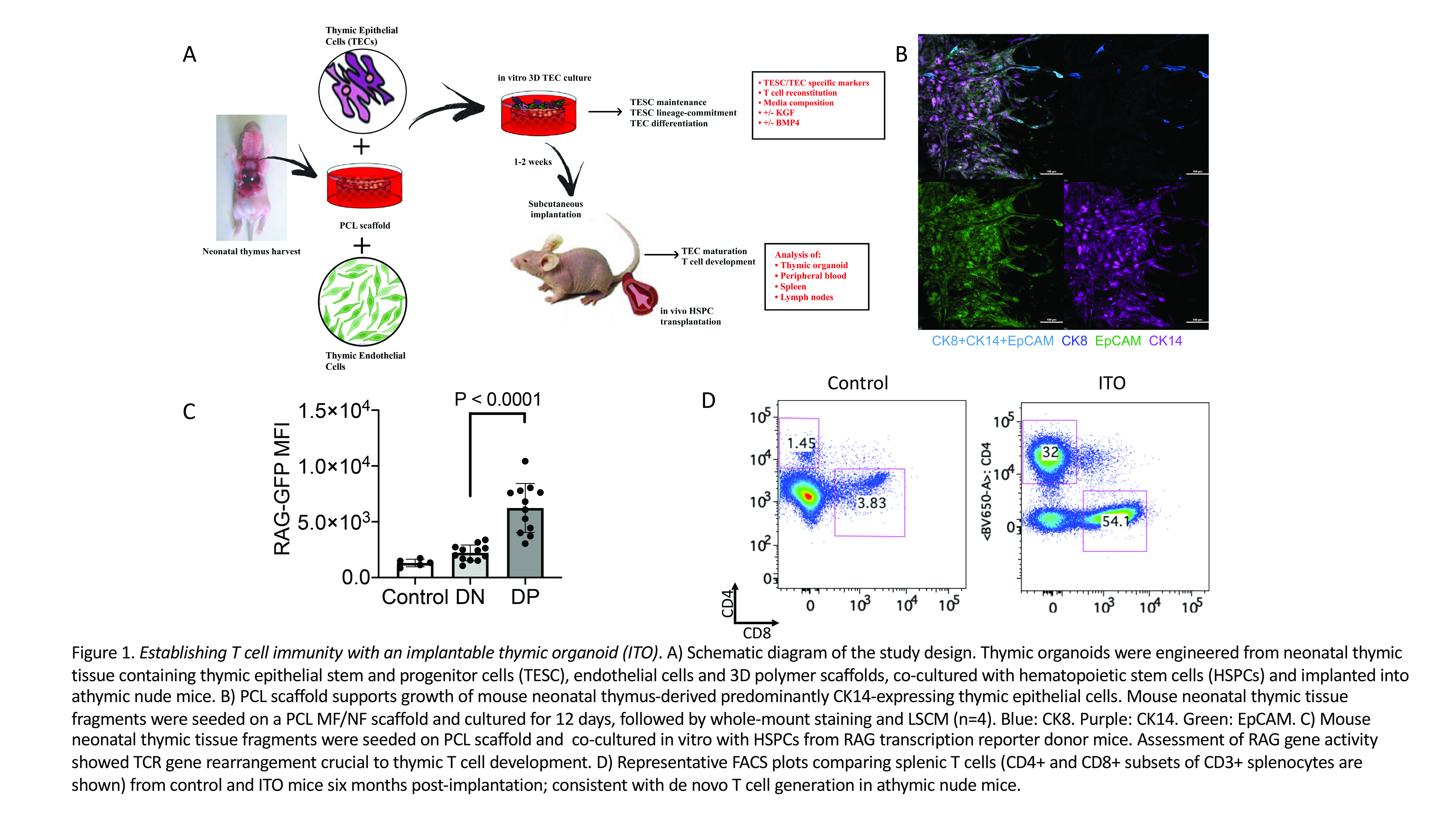
We designed and tested organoids based on 3D scaffolds fabricated by electrospinning from the FDA-approved biopolymer polycaprolactone (PCL), mouse neonatal thymic tissue fragments containing thymic epithelial cells including epithelial progenitor cells, and functional primary thymic endothelial cells that were engineered with the adenovirus E4ORF1 gene promoting long-term survival while preserving regenerative potential of endothelial cells. Hematopoietic stem and progenitor cells (HSPCs) were seeded to facilitate lymphostromal interactions and sustained functionality both in vitro and in vivo.
Organoid characterization involved laser scanning confocal microscopy for thymic epithelial cell specific markers, live cell imaging, and multicolor flow cytometric analysis of epithelial cells and developing T cell precursors. Our 3D culture system supported growth and expansion of primary thymic epithelial cells for at least 4 weeks (confirmed by live cell microscopy with FoxN1 lineage tracing), in contrast to the historic experience with 2D culture of primary thymic epithelial cells that is not sustainable for more than 24 to 48 hours. Immunofluorescence analysis of thymic organoids revealed that thymic epithelial cell populations included CK8/CK14 double positive thymic epithelial progenitor cells. Mature thymic epithelial cells displayed a predominantly medullary thymic epithelial cell phenotype (CK8âCK14+) (Figure 1B). We optimized the culture conditions by evaluating the impact of the thymic growth factors, bone morphogenetic protein 4 (BMP4) and keratinocyte growth factor (KGF), and the addition of defined numbers of thymic endothelial cells, a cell type that was previously found to boost thymic regeneration. Media supplementation with BMP4 and KGF promoted growth of thymic epithelial cells in vitro. Co-culture of thymic organoids with HSPCs induced their differentiation through successive CD4-CD8- (double negative), CD4+CD8+ (double positive) and CD4+CD8-CD3+ or CD8+CD4-CD3+ (single positive) stages. T cell receptor (TCR) gene rearrangement is a hallmark of thymic T cell development, and this was confirmed by assessment RAG gene activity in experiments utilizing HSPCs of RAG transcription reporter mice (Figure 1C). The addition of thymic endothelial cells to the organoid further enhanced T cell development in vitro.
In vivo studies included subcutaneous implantation of the optimized thymic organoid systems into athymic mice, along with co-cultured luciferase-transgenic HSPCs. The fate of ITOs was studied via bioluminescence imaging as well as immunophenotyping of lymphoid tissues via flow cytometry, histology and immunohistochemistry to assess both T cell reconstitution and biomaterial responses. Our data indicate that ITOs established de novo T cell generation in athymic mice and partially recapitulated thymic function (Figure 1D), and similar to our in vitro findings, expansion of developing T cells within the ITO was enhanced by thymic endothelial cells.
We conclude that thymus-independent in vivo T cell generation in a tissue-engineered ITO is feasible, and further development of this technology is expected to produce an exciting innovative strategy for the treatment of T cell deficiency.