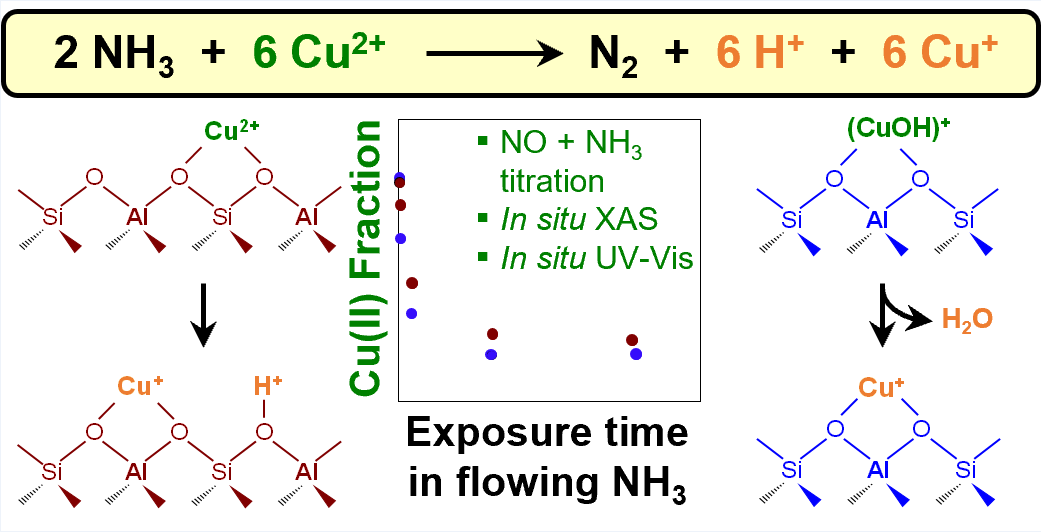
Cu-Exchanged zeolites catalyze various redox reactions including the selective catalytic reduction (SCR) of NO
x with NH
3 and the partial oxidation of methane. Reduction of Cu(II) cations to Cu(I) by NH
3 alone has been reported in the literature, yet mechanistic details such as the reaction stoichiometry and Cu site requirements remain incompletely understood. This is an undesired side-reaction that occurs during steady-state NO
x SCR and can unintentionally influence SCR-relevant spectroscopic or titrimetric characterization experiments. Here, we synthesize model Cu-exchanged chabazite (Cu-CHA) zeolites with varying densities of Cu ions and varying distributions of mononuclear Cu(II) site types (Cu
2+, (CuOH)
+). We investigate NH
3-assisted Cu(II) reduction reactions through a combination of titrimetric and spectroscopic measurements to quantify changes to Cu oxidation states and the concomitant formation of gas-phase reaction products (Figure 1). The fraction of Cu(II) sites reduced by NH
3 alone in a given time period is quantified through subsequent complete reduction to Cu(I) in an NO + NH
3 gas mixture, corroborated by
in situ UV-visible and X-ray absorption spectroscopies. We show that both mononuclear Cu(II) site types are able to reduce in NH
3 alone, and with similar apparent kinetics. NH
3 temperature programmed reduction (TPR) shows that NH
3-assisted reduction of Cu(II) sites forms approximately one N
2 molecule per 6 Cu sites, regardless of Cu-CHA sample composition, implying that two NH
3 molecules react with six Cu(II) species to produce one N
2 molecule and six Cu(I) species. Our findings provide insights into the reaction pathways by which NH
3 alone reduces mononuclear Cu(II) sites in zeolites, which should be considered in both the design and investigation of NO
x SCR catalysts.