Humins are carbonaceous, polymeric by-products formed during acid-catalyzed reactions of biomass-derived species. Such by-products cause significant carbon losses in the feed and can cause catalyst deactivation. Investigating humins formation is challenging due to their complex and unknown molecular structure. Likewise, during the condensed phase catalytic conversion of biomass, several reactions occur simultaneously, making it difficult to isolate humins formation reactions
1. Analysis of IR spectra has given insights about the functional groups present in humins and thereby suggesting possible reaction mechanisms
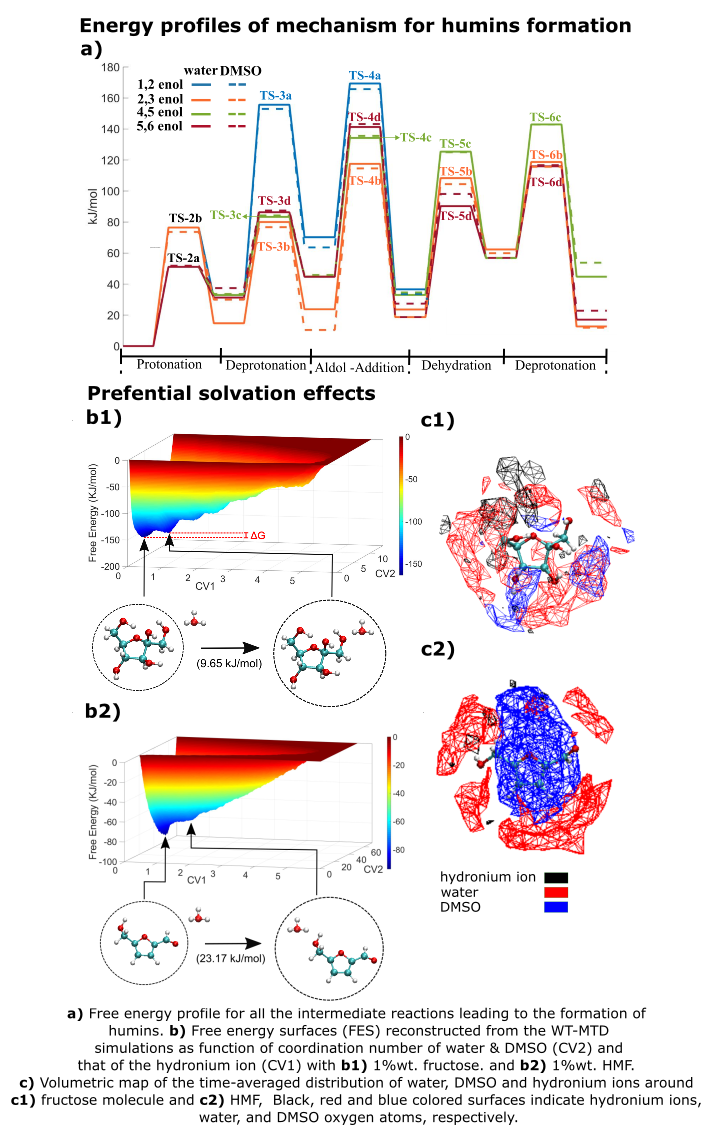
2. Also, modification of the solvent environment by the addition of aprotic solvents, have shown to suppress humins formation. However, mechanistic understanding of the humins formation process is not known to develop strategies to avoid their formation. The role of solvents in humins formation is investigated in this study at two different levels: physical interactions (preferential solvation of reacting species) and chemical interactions (altering reaction kinetics and thermodynamics). Molecular dynamics simulations and well-tempered metadynamics are performed revealing how the interaction of the acid catalyst with biomass moieties is altered in the presence of an aprotic solvent. The solvent also preferentially solvates biomass molecules and their derivatives providing a shielding effect for their degradation to humins formation
3. To analyze the chemical role of solvent, key steps in the reaction pathways (previously identified using DFT computations) leading to humins formation are further analyzed in explicit solvent environment using ab initio molecular dynamics and the novel ReSOLV method
4. These computations reveal the effect of solvent environment in altering reaction free energies and kinetic barriers for humins formation in the condensed phase. We believe that these findings can aid in designing novel catalytic systems, solvents, and operation conditions to minimize humins formation.
- ChemSusChem 2013, 6, 1651 â 1658
- Energy Fuels 2011, 25, 4745â4755
- ChemPhysChem 2021,22, 2222-2230
- Chem Eng, 2022