2022 Annual Meeting
(7h) Influence of Interactions between Solvent Structures and Reactant Alkyl Groups on Lewis-Acid Catalyzed Epoxidations
Authors
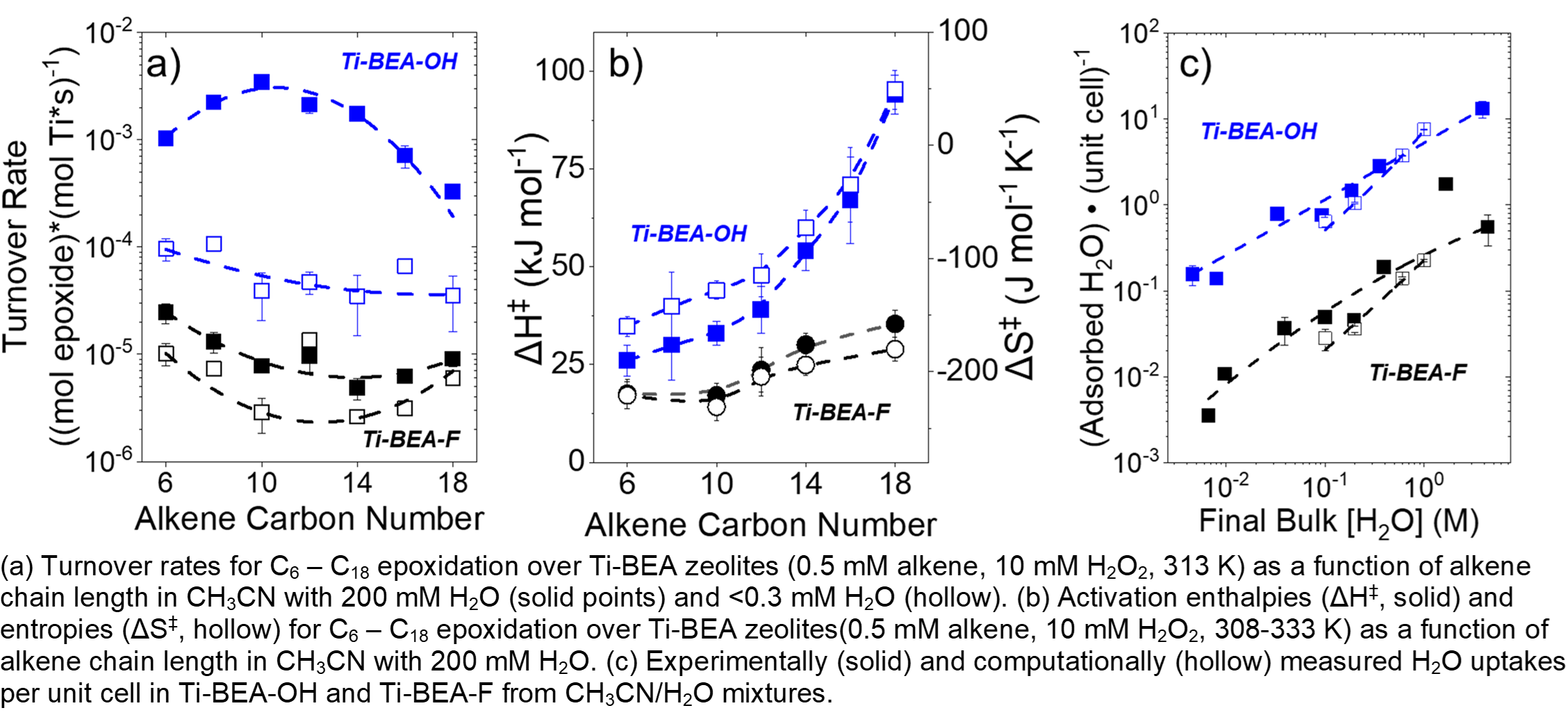
Epoxidation rates are 40-450 times greater across the range of alkenes over hydrophilic Ti-BEA-OH than hydrophobic Ti-BEA-F with 200 mM H2O present. Adsorption measurements with 1H NMR and corresponding results from grand canonical molecular dynamics simulations (GCMD) show that Ti-BEA-OH adsorbs 10-20 times more H2O than Ti-BEA-F. Rates decrease for each catalyst under anhydrous conditions, but Ti-BEA-OH shows greater rate decreases (10-90 times) than Ti-BEA-F (< 3 times). The rate differences do not result from changes in reaction mechanism or mass transfer constraints, but rather changes in transition state stability described by activation enthalpies (ÎHâ¡) and entropies (ÎSâ¡). ÎHâ¡ and ÎSâ¡ increase systematically with chain length over each zeolite because longer alkyl chains disrupt more solvent molecules. In addition, disruption of H2O in Ti-BEA-OH yields greater changes in ÎHâ¡ and ÎSâ¡ than for CH3CN, which dominates the pores of Ti-BEA-F. Corresponding epoxide adsorption enthalpies, measured experimentally and computed with GCMD, become more endothermic as chain length increases and correlate to ÎHâ¡, providing evidence for chain length dependent disruption of solvent molecules. These findings show that the entropic gain per enthalpic cost of solvent disruption varies with alkene chain length, silanol density, and more directly with the composition of the solvent within the pore. Together, these changes can be used to increase epoxidation turnover rates.