Breadcrumb
- Home
- Publications
- Proceedings
- 2022 Annual Meeting
- Catalysis and Reaction Engineering Division
- Microporous and Mesoporous Materials I: Catalytic Sites
- (7g) Mechanistic Analysis of CO Oxidation over Mixed-Valence Oxo-Bridged Trimers

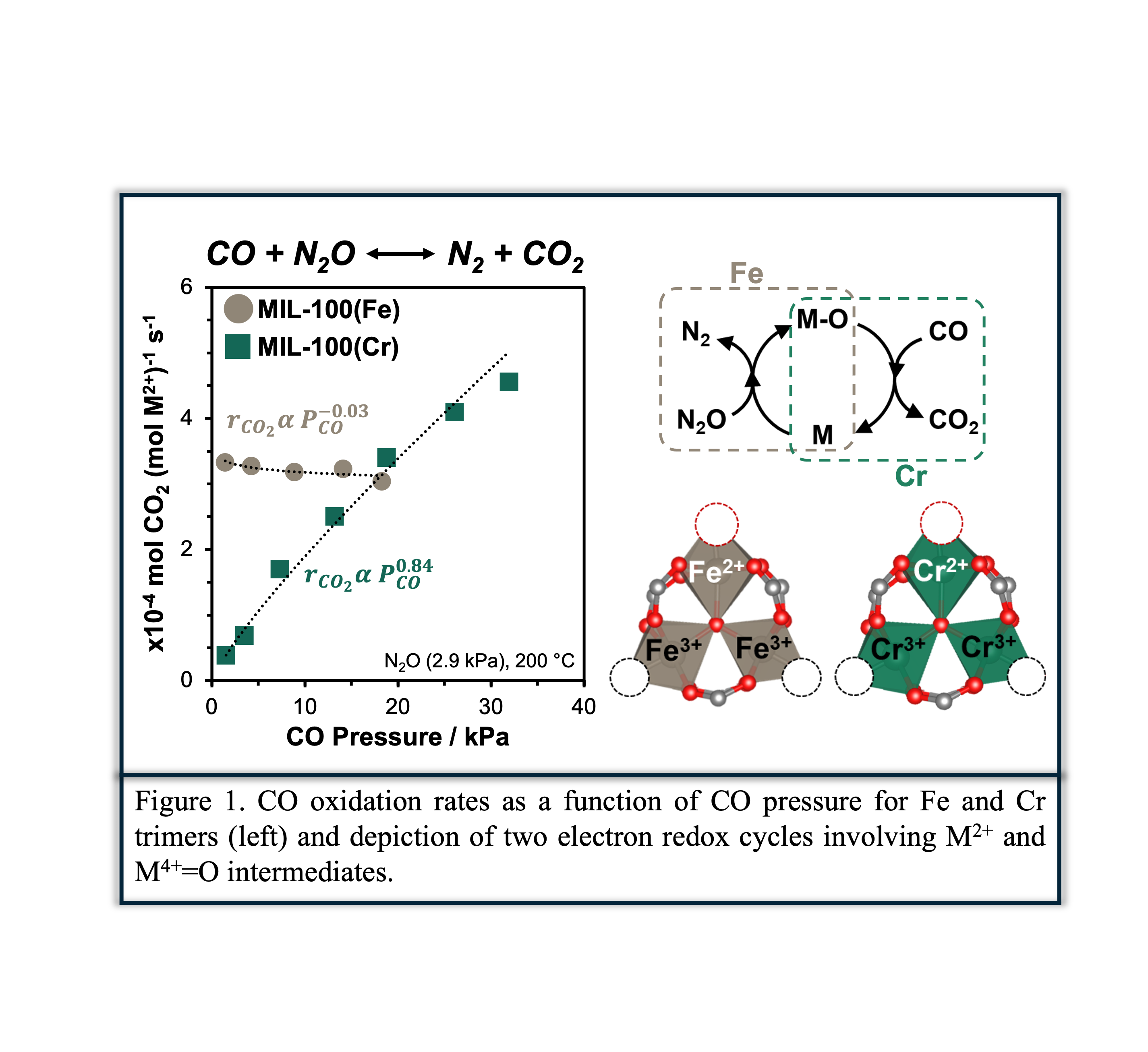
Steady state and transient kinetic experiments combined with isotopic tracer studies suggest steps constituting the oxidation half cycle as being kinetically relevant over both Fe and Cr trimers, with activation energies reflecting standard state free energy differences between Fe(IV)=O intermediates and gas phase nitrogen, and the bare Fe2+ surface and gas phase N2O. Cr trimers enable access to much greater coverages of metal-oxo intermediates, reflected in lower sensitivities to CO pressure relative to their Fe trimer counterparts (Figure 1). Overall, these results provide unprecedented levels of clarity as to the identity and kinetic relevance of steps mediating oxidative turnovers over multinuclear metal-oxo clusters, as well as the utility of metal organic framework materials in addressing questions hitherto rendered intractable by heterogeneity in active site speciation.