2022 Annual Meeting
(706a) 3D Printed Hybrid Monolith for CO2 Capture
Authors
To ensure an efficient adsorption process, the properties of the used adsorbents must be optimized. Nowadays, traditional packed beds with pellets/beads are still used in industry. However, this type of structured adsorbent possess some drawbacks, like a high-pressure drop and attrition. This way, additive manufacturing (AM), also known as 3D-printing, emerges as an alternative to develop structured adsorbents suitable for large-scale applications. The shaping of adsorbent materials using AM technologies has still a long path of exploration.
In this work, an adsorbent monolith consisting of 50 %wt. of zeolite 13X and 50 %wt. of activated carbon was developed using direct ink writing. To evaluate the suitability of this material for large-scale applications, the impact of shaping by direct ink writing of a structured adsorbent material for CO2 capture on the characteristics of the material and mechanical strength were analyzed. The material was characterized by employing N2 physisorption at 77 K, CO2 adsorption at 273 K, mercury porosimetry, and crushing tests.
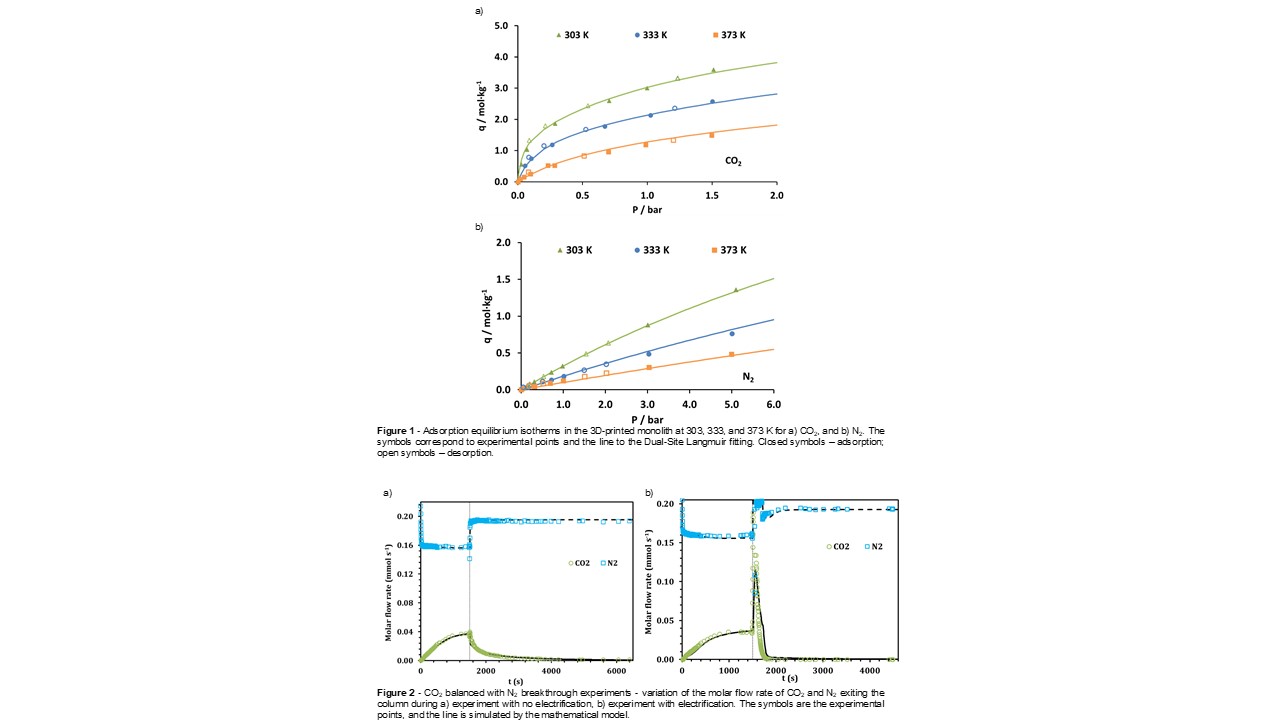
Additionally, pure CO2 and N2 adsorption equilibrium isotherms were measured at 303, 333, and 373 K and in a pressure range of 0 to 1.5 bar and 0 to 5 bar, respectively â Figure 1. The isotherms were fitted and well described with the Dual-Site Langmuir model. CO2 is the gas with a higher affinity towards the 3D-printed monolith. Considering a CO2 molar fraction of 0.15, at 1 bar and 303 K, the selectivity of the monolith is 30.7.
Dynamic adsorption tests were also performed with and without the assistance of electric current to regenerate the material. The CO2/N2 binary breakthrough curves for these two runs are displayed in Figure 2. A mathematical model was utilized to describe the behavior observed in the breakthrough curves, and the simulation outcomes can also be examined in Figure 2. A compressive CO2 mass front is seen in the adsorption step and a dispersive mass front in the regeneration step, which corroborates with the behavior of the measured isotherms â Figure 1. The desorption is faster when the electrification of the adsorbent material is performed (compare Figure 2 a) and b)).