2022 Annual Meeting
(688d) Computational Fluid Particle Dynamics Illuminates Developmental Anatomical Feature Influence on Aerosol Deposition Patterns in 6-Year-Old Upper Airway CT-Scan Models
Authors
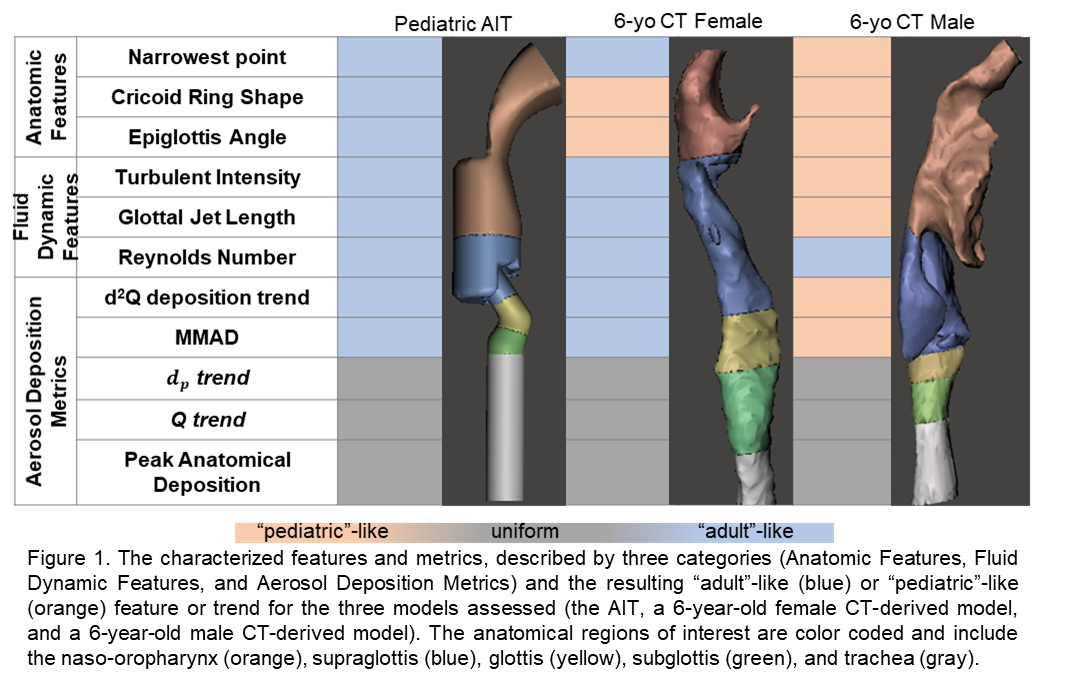
In addition to these characterizations, the resultant aerosol deposition pattern and metrics of interest were evaluated for idealized model particles and an Advair® Diskus® aerosol mimic model.4 Some aerosol deposition trends were consistent among all three models, such as a significant increase in deposition as a function of particle size (100 nm -10 μm) and an insignificant relationship across flow rates (10 Lpm â 120 Lpm). For both the impaction parameter (d2Q) and the Advair® Diskus® mass median aerodynamic diameter (MMAD) there was agreement between the AIT and the female CT-scan model, both of which presented with predominantly âadultâ-like features. This suggests that the AIT would be better at predicting subjects with a more developed upper airway displaying more âadultâ-like features. This work demonstrates the transient development of upper airways in pediatric subjects and the resultant variation in aerosol deposition patterns and metrics that can be uniform across subjects, independent of development, or predictable from âadultâ-like idealized models. We have quantified and demonstrated differences between adult and pediatric upper airways that highlight the need to recognize and integrate pediatric aerosol requirements into inhalable therapeutics.
REFERENCES:
- Ahookhosh, K.; Pourmehran, O.; Aminfar, H.; Mohammadpourfard, M.; Sarafraz, M. M.; Hamishehkar, H., Development of human respiratory airway models: A review. European Journal of Pharmaceutical Sciences 2020, 145, 105233.
- Di Cicco, M.; Kantar, A.; Masini, B.; Nuzzi, G.; Ragazzo, V.; Peroni, D., Structural and functional development in airways throughout childhood: Children are not small adults. Pediatric Pulmonology 2021, 56 (1), 240-251.
- Golshahi, L.; Finlay, W., An idealized child throat that mimics average pediatric oropharyngeal deposition. Aerosol Science and Technology 2012, 46 (5), i-iv.
- Weers, J.; Clark, A., The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharmaceutical research 2017, 34 (3), 507-528.