2022 Annual Meeting
(682b) Electrochemical Conversion of Low Concentration CO2 Gas in a Membrane Electrode Assembly Electrolyzer
Authors
Ung Lee, Korea Institute of Science and Technology (KIST)
Yun Jeong Hwang, Seoul National University
Dongjin Kim, Korea Institute of Science and Technology
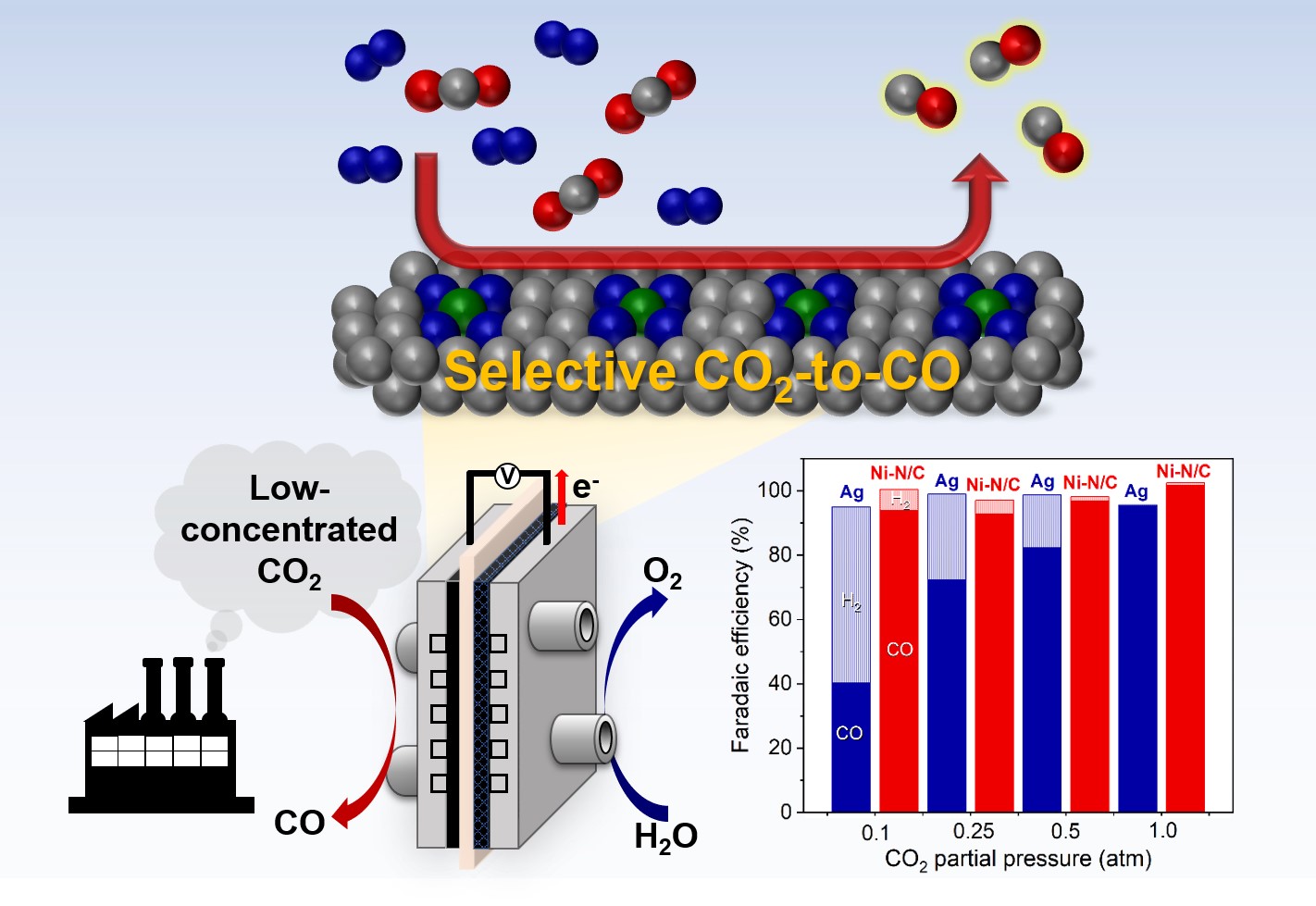
Electrochemical CO2 reduction to valuable chemicals is a promising technology for mitigating the global climate crisis and developing new processes for chemical production. The recent development of an electrolyzer gave a remarkable advancement of CO2 reduction performance in production rate and selectivity. In particular, a membrane electrode assembly (MEA) electrolyzer whose electrodes and membrane are assembled without a catholyte has been studied as a promising configuration for stackable applications. To increase the practical applicability of the CO2 reduction system, the direct conversion of low concentrations of CO2 is an essential approach due to the expensive gas conditioning process for pure CO2, which is required for reactant for electrochemical CO2 reduction. In this research, we explored the CO2 reduction with various CO2 concentrations in the MEA system and found that suppressing the hydrogen evolution reaction (HER) became more critical at low concentrations. We demonstrated that a Ni single-atom catalyst produced CO with high selectivity under various concentrations of CO2. When the partial pressure of CO2 lowered from 1.0 to 0.1 atm, Ni-N/C maintained >93% of CO Faradaic efficiency, but Ag nanoparticle showed a decrease CO Faradaic efficiency from 94% to 40%. We further developed extrinsic operating conditions controlling the water transfer inside of MEA electrolyzer and consequently, improved CO selectivity on the basis of a computational fluid dynamics simulations.