2022 Annual Meeting
(618g) In Situ CO2 Capture and Catalytic Hydrogenation at Ni/Alkaline Earth Carbonate Interfaces
Authors
Wen Liu, Nanyang Technical University
Ribooga Chang, Uppsala University
Ocean Cheung, Stockholm University
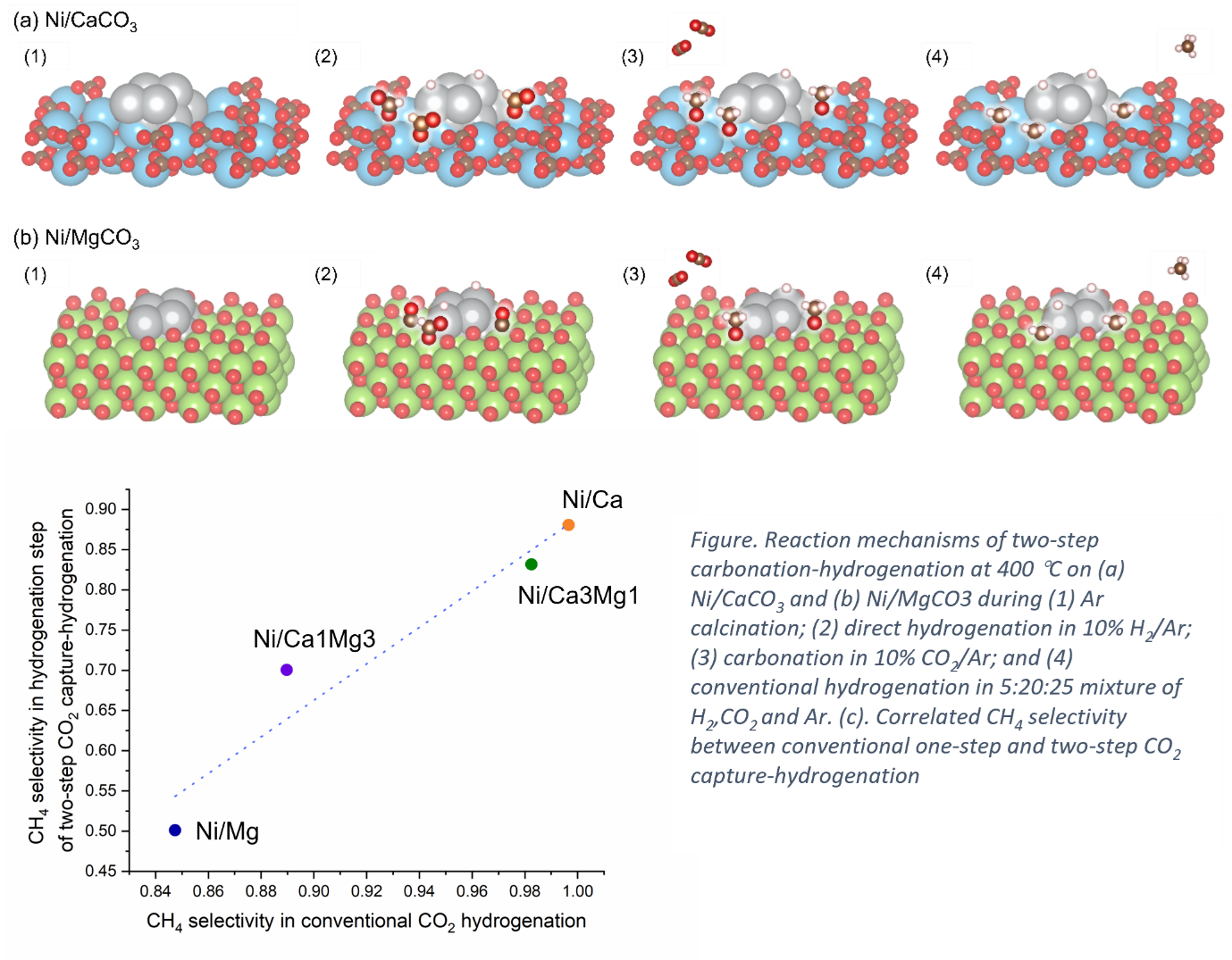
CO2 capture and utilization (CCU) is a promising strategy to effectively mitigate the adverse greenhouse effects caused by anthropogenic CO2 emissions. Dual function materials (DFMs) combining metal catalysts with alkaline earth metal oxide CO2 adsorbents offer a potential to integrate CO2 capture with conversion through a cyclic CO2 capture-hydrogenation scheme, thus achieving process intensification. We report the study of DFMs consisting of Ni nanoparticles supported on MgCO3, CaCO3 or a mixture of both, for CO2 capture and the subsequent hydrogenation at 400 °C. The results show that the Ni/carbonate interfaces could be hydrogenated in two ways, depending on the nature of the carbonate. First, unstable carbonates could be decomposed to release gaseous CO2, which is subsequently adsorbed and hydrogenated on Ni. Alternatively, stable carbonates could be directly hydrogenated at the Ni/carbonate interface. In situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS), shows that the direct hydrogenation of carbonates proceeds via the formate pathway, whereas the gaseous hydrogenation proceeds via both the formate pathway and the reverse water-gas-shift (RWGS pathway). Interestingly, for any specific DFM, the reaction pathway and product selectivity during the CO2 capture-hydrogenation cycles are strongly correlated to those during a conventional CO2 hydrogenation process, in which Ni/CaCO3 shows the highest CO2 conversion (80%) and CH4 selectivity (>99.5%) via the formate pathway, at 400 °C, 1 bar total pressure. In this sense, the two-step capture-hydrogenation process is not only an integrated CO2 capture and upgrading scheme, but also an experimental approach for elucidate CO2 reaction mechanisms.