Atomic understanding of interfaces provides guidance toward the modeling and design catalysts with improved activity, selectivity, and stability. A comprehensive understanding on how the metal-electrolyte interface behaves is critical for predicting, designing, and improving current commercial technologies and opening doors for the development of future technologies. Hydrogen fuel cells that utilize platinum typically operate between 0.6 and 1.0 V versus the reversible hydrogen electrode (V
RHE) [1], which is the potential range where Pt oxidation to PtOH adlayer species dominate in HClO
4. We discuss how experiments and density functional theory (DFT) computations led us to discover that platinum, a noble metal that is frequently utilized as a catalyst in the cathode of fuel cells, restructures when the voltage is held constant between fuel-cell relevant voltages of 0.6 and 1.0 V on a reversible hydrogen electrode scale (V
RHE).
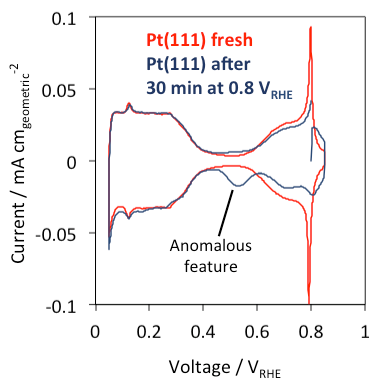
An anomalous reduction feature at ~0.53 VRHE was observed on a Pt(111) single crystal in Ar-saturated HClO4 after holding at the fuel-cell relevant voltage of 0.8 VRHE (Figure 1). Decades of research has established that Pt(111) in HClO4 oxidizes H2O to adsorbed *OH between 0.6 and 1.0 VRHE [2-4] and this current model is unable to explain the anomalous feature. Using a combination of computational, electrochemical, spectroscopic, and imaging probes, we find that chronoamperometry at voltages between 0.6 and 1.0 VRHE results in a mildly-roughened Pt(111) surface [5], presumably due to an *OH-induced release of surface stress. The catalytic performance of this mildly roughened Pt(111) was tested for the oxygen reduction reaction (ORR) and carbon monoxide oxidation (CO Oxidation) where it was found that the ORR rate is seemingly structure insensitive and CO Oxidation rate is surprisingly structure sensitive. Overall, this discovery demonstrates the importance of understanding how dynamic and steady operating conditions influence the electrode-electrolyte interface.
References
1. The Hydrogen and Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration (MYRD&D) Plan, Section 3.4 Fuel Cells, S. Dep. Energy. (2017).
2. Al Jaaf-Golze, D.M. Kolb, D. Scherson, J. Electroanal. Chem. 200 (1986) 353â362.
3. T.M. Koper, J.J. Lukkien, Surf. Sci. 498 (2002) 105â115.
4. H. Kristoffersen, K. Chan, T. Vegge, H.A. Hansen, Chem. Commun. 56 (2020) 427â430.
5. Komanicky, K.C. Chang, A. Menzel, N.M. MarkoviÄ, H. You, X. Wang, D. Myers, J. Electrochem. Soc. 15 (2006) B446âB451.