2022 Annual Meeting
(559f) A Facile, Catchall Pathway for Carbon Impurity Minimization Via Ligand Engineering of Colloidal Cu(InxGa1-x)S2 Nanoparticles for Thin-Film Photovoltaics
Authors
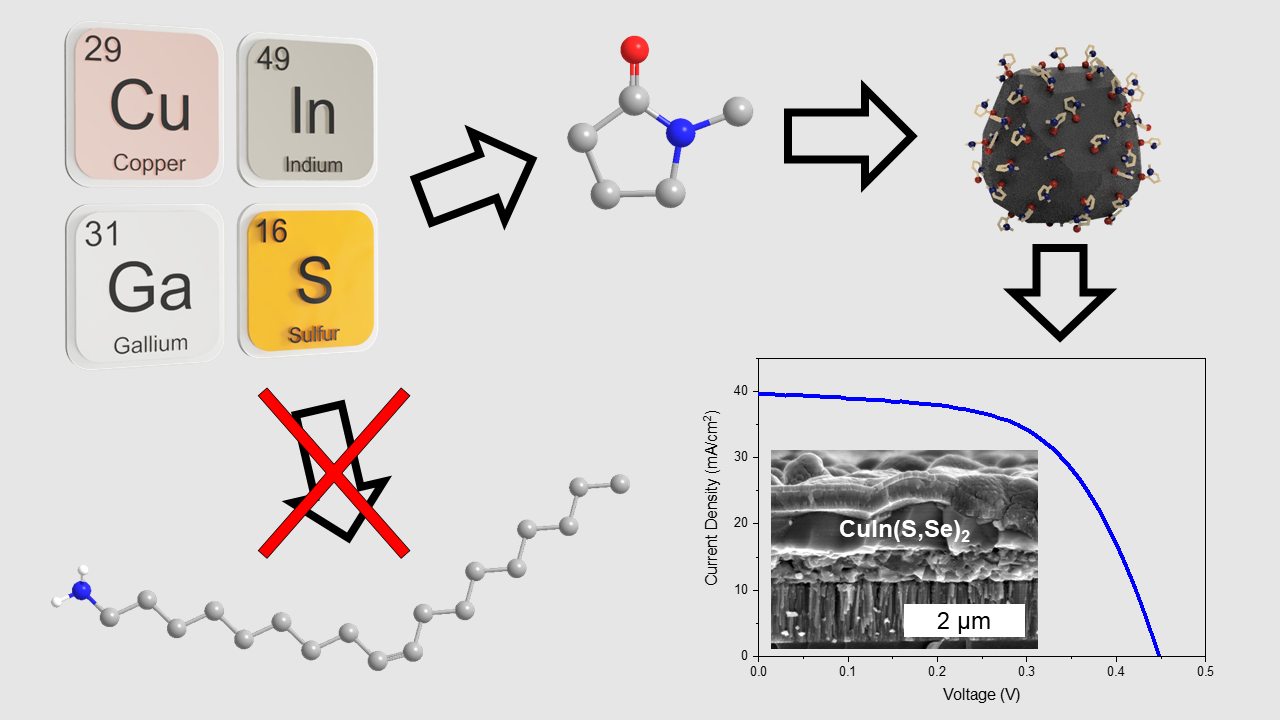
Solution-processing of chalcogenide thin-films is usually broken down into two main pathways for creating inks of the desired materials: the molecular precursor route,2â4 which involves direct dissolution of elemental precursors, and the colloidal nanoparticle (NP) route,5â7which involves synthesis of colloidal semiconductor NPs and will be the focus of this presentation. Traditionally, the colloidal NP route has been used as a reliable way of depositing desired materials without worry of substantial impurities from precursor salts that may be present via the molecular precursor route. Additionally, colloidal NP inks usually have simpler deposition procedures due to higher concentration inks allowing for fewer steps. To synthesize these NPs, species like trioctylphosphine, oleylamine (OLA), or dodecanethiol often serve as stabilizing surface ligands and kinetic modifiers to carefully control the shape and size of the NPs.8 Although these species work exceedingly well in doing this, they leave substantial amounts of carbon impurities in films after NP coatings, due to their low volatility.9â11 This leads to a coke-like residue that remains trapped in the film in a fine-grain layer, harming performance. These carbon residues are nearly impossible to remove without significant changes to the reaction chemistry. Ligand-exchange methods can replace these long-chain ligands post-synthesis, but the cost-advantage that solution-processing aims to achieve over vacuum processing is diminished due to the high solvent usage of such a tedious process. Furthermore, this ligand exchange method can still show lingering carbon residues due to incomplete exchange.11
To this end, we studied the industrially-relevant solvent, N-methyl-2-pyrrolidone (NMP) in great detail as an alternative reaction medium and ligand for colloidal NP synthesis of CIGSSe. NMP was chosen for its more favorable properties including its higher volatility which aids in its removal from the film, lowering the chance for lingering carbon residues during. In addition to serving as the primary surface ligand, NMP is also used as the suspension medium for exceptionally stable colloidal inks up to 200 mg/mL that remain suspended in excess of 6 months. Films of NMP-capped CuInSe2 are shown to exhibit a substantially lower carbon content than that of films prepared via OLA-capped CuInSe2 owing to the higher volatility of a smaller species like NMP. The potential for fabricating devices from these solution processed NMP-capped NPs was also investigated. Preliminary results show that CuIn(S,Se)2 devices can produce power conversion efficiencies in excess of 10% with a short-circuit current (JSC) of 40.0 mA/cm2, a comparable JSC to that of vacuum-processed devices.12Morphological studies of these films via scanning-electron microscopy (SEM) in tandem with data from Raman spectroscopy support the idea that the identity of the fine-grain layer formed during fabrication is characteristically different than that from traditional, non-hydrazine pathways, indicating a higher-quality absorber regardless of the existence of a fine-grain layer. Work is still ongoing to fabricate high-quality device films of gallium-containing CIGSSe in order to achieve high-efficiency devices. Preliminary results show a very high photoluminescence (PL) response for CIGSSe films fabricated from NMP-capped CIGS NPs. With optimizations to nanoparticle syntheses and coating procedures, high-efficiency CIGSSe devices should be obtainable.
References
- Zhang T, Yang Y, Liu D, et al. High efficiency solution-processed thin-film Cu(In,Ga)(Se,S) 2 solar cells. Energy & Environmental Science. 2016;9(12):3674-3681. doi:10.1039/C6EE02352E
- Zhao X, Deshmukh SD, Rokke DJ, et al. Investigating Chemistry of Metal Dissolution in AmineâThiol Mixtures and Exploiting It toward Benign Ink Formulation for Metal Chalcogenide Thin Films. Chemistry of Materials. 2019;31(15):5674-5682. doi:10.1021/acs.chemmater.9b01566
- Mitzi DB, Yuan M, Liu W, et al. A High-Efficiency Solution-Deposited Thin-Film Photovoltaic Device. Advanced Materials. 2008;20(19):3657-3662. doi:10.1002/adma.200800555
- Zhao Y, Yuan S, Chang Q, et al. Controllable Formation of Ordered Vacancy Compound for High Efficiency Solution Processed Cu(In,Ga)Se 2 Solar Cells. Advanced Functional Materials. 2021;31(10):2007928. doi:10.1002/adfm.202007928
- Guo Q, Ford GM, Agrawal R, Hillhouse HW. Ink formulation and low-temperature incorporation of sodium to yield 12% efficient Cu(In,Ga)(S,Se) 2 solar cells from sulfide nanocrystal inks. Progress in Photovoltaics: Research and Applications. 2013;21(1):64-71. doi:10.1002/pip.2200
- McLeod SM, Hages CJ, Carter NJ, Agrawal R. Synthesis and characterization of 15% efficient CIGSSe solar cells from nanoparticle inks. Progress in Photovoltaics: Research and Applications. 2015;23(11):1550-1556. doi:10.1002/pip.2588
- Ahn S, Rehan S, Gwon Moon D, et al. An amorphous CuâInâS nanoparticle-based precursor ink with improved atom economy for CuInSe2 solar cells with 10.85% efficiency. Green Chemistry. 2017;19:1268. doi:10.1039/c6gc03280j
- Coughlan C, Ibáñez M, Dobrozhan O, Singh A, Cabot A, Ryan KM. Compound copper chalcogenide nanocrystals. Chemical Reviews. 2017;117(9):5865-6109. doi:10.1021/acs.chemrev.6b00376
- McLeod S, Alruqobah E, Agrawal R. Liquid assisted grain growth in solution processed Cu(In,Ga)(S,Se)2. Solar Energy Materials and Solar Cells. 2019;195:12-23. doi:10.1016/j.solmat.2019.02.020
- Deshmukh SD, Weideman KG, Ellis RG, Kisslinger K, Agrawal R. Enabling fine-grain free 2-micron thick CISe/CIGSe film fabrication via a non-hydrazine based solution processing route. Materials Advances. Published online 2022. doi:10.1039/D2MA00095D
- Ellis RG, Turnley JW, Rokke DJ, et al. Hybrid Ligand Exchange of Cu(In,Ga)S 2 Nanoparticles for Carbon Impurity Removal in Solution-Processed Photovoltaics. Chemistry of Materials. Published online June 4, 2020:acs.chemmater.0c00966. doi:10.1021/acs.chemmater.0c00966
- Suresh S, Uhl AR. Present Status of SolutionâProcessing Routes for Cu(In,Ga)(S,Se) 2 Solar Cell Absorbers. Advanced Energy Materials. 2021;2003743:2003743. doi:10.1002/aenm.202003743