2022 Annual Meeting
(533q) Cationic Dye Adsorption on Metal Organic Framework: An Equilibrium Study
Authors
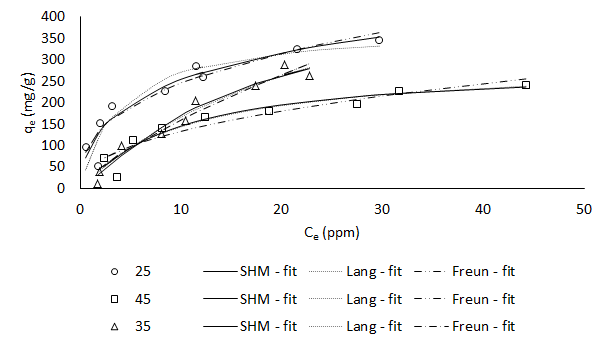
In the present work, a functionalized MOF (MIL-101-SO3H1) was synthesized hydrothermally and characterized for its physico-chemical properties such as BET surface area, morphology, surface composition and point of zero-charge. MIL-SO3H (MILS) was used to adsorb a cationic dye viz. basic yellow. Optimal conditions for enhanced equilibrium capacities were identified using Design of Experiments (DoE). The adsorption capacity was found to be 320 mg/g at pH 6, temperature 25 °C, and dosage of 1 g/L. The equilibrium data was fitted to Simple Hillâs Model (SHM), a statistical physics-based model which gives considerable insight into the interaction between adsorptive and adsorbent. The adsorption process was found to be exothermic with enthalpy of adsorption of -29.37 kJ/mol. The adsorption mechanism was revealed to be an electrostatic interaction between the cationic dye and the SO3- group present on the functionalized MOF. Kinetic studies were also carried out with this MOF at the optimal conditions.
References:
- Ma, L., Xu, L., Jiang, H. & Yuan, X. Comparative research on three types of MIL-101(Cr)-SO 3 H for esterification of cyclohexene with formic acid. RSC Adv. 9, 5692â5700 (2019).